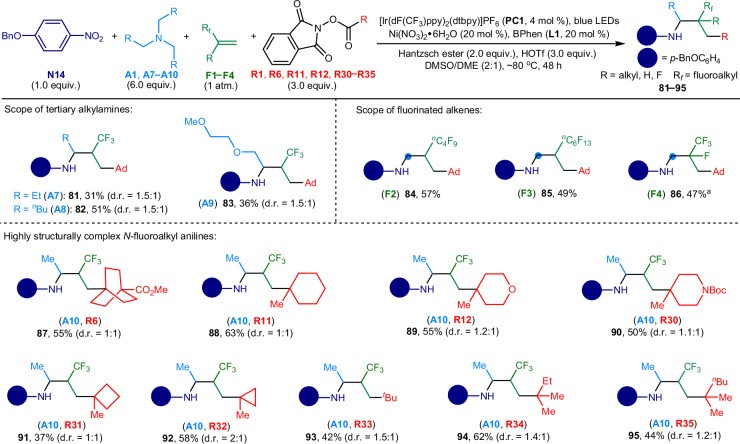
Fig. 5. Scope of tertiary alkylamines and fluoro-based alkenes.
General reaction conditions: nitroarene (N14, 1.0 equiv., 0.15 mmol), tertiary alkylamine (A1, A7 − A10, 6.0 equiv., 0.90 mmol.), fluoro-substituted propene (F1, F4: 1 atm.; F2, F3: 20 equiv.), redox-active ester (R1, R6, R11, R12, and R30 − R35, 3.0 equiv., 0.45 mmol), PC1 (4 mol %), Ni(NO3)2•6H2O (20 mol %), bathophenanthroline (BPhen, L1: 20 mol %), Hantzsch ester (2.0 equiv., 0.30 mmol), HOTf (3.0 equiv., 0.45 mmol), DMSO/DME (v/v = 2:1, 6 mL), ~80 oC, blue LEDs (30 W, 456–460 nm), 48 h. Unless otherwise noted, A1, F1 and R1 were used as reaction substrates. aThe reaction was conducted at 40 oC for 24 h. Ad, 1-adamantyl; Me, methyl; Boc, tert-butyloxycarbonyl, tBu, tert-butyl; Et, ethyl, nBu, n-butyl.