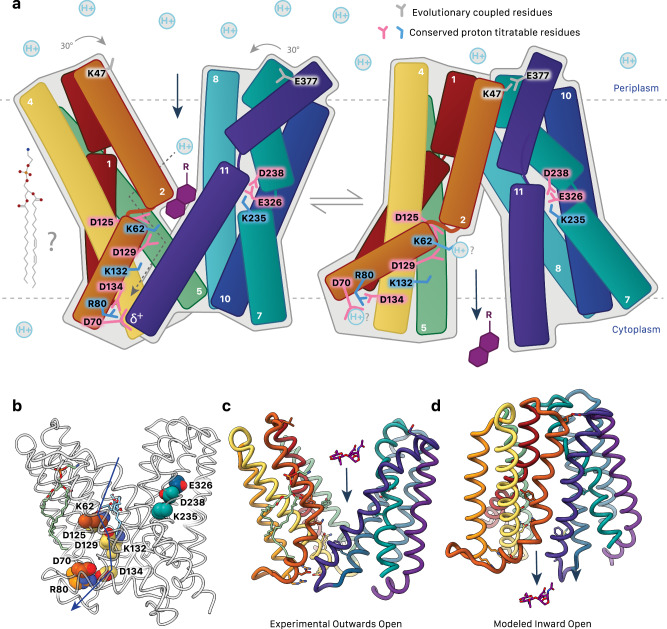
Fig. 7. Proposed AmpG transport mechanism.
a Substrate binding (GlcNAc-1,6-anhydroMurNAc with R representing -OH, tri, tetra, and pentapeptide chains; purple) and protonation of conserved acidic residues are proposed to result in a rigid body conformational shift of AmpG. Motif A Asp70 interacts with the TM11 helix dipole to stabilize the outward open state. The disruption of this interaction e.g., by protonation is proposed to be a key part of the switch to the inward conformation. Positions of conserved titratable residues shown. Dotted arrows suggest a proposed flow of proton transport. Evolutionary coupled residues Lys47 and Glu377 are over 30 Å apart in the outward model but come together to interact in the inward conformation. A potential role of lipid(s) in this mechanism, as suggested by the observed ordered PE, is yet to be determined. b Conserved titratable residues as in a shown as spheres. c Experimentally determined outward-open structure of AmpG. A GlcNAc-1,6-anhydroMurNAc substrate is shown in sticks. d Modeled inward conformation.