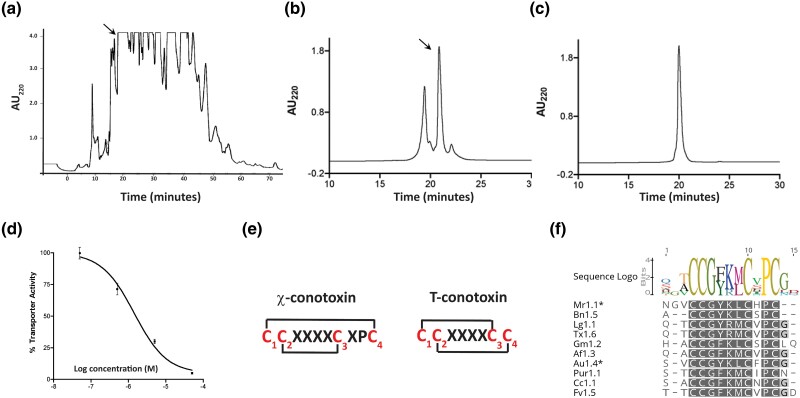
Fig. 2.
χ-Conopeptide is highly expressed in the venom of C. aulicus and these peptides form a structurally and pharmacologically distinct family in the T-superfamily of conotoxins. a) HPLC chromatogram showing the absorbance profile at 220 nm of crude C. aulicus venom fractionated on a preparative C18 column using a linear gradient of 1.3% B90/min at a flow rate of 20 mL/min. The fraction marked by the arrow exhibited activity against hNET, as described in the Results section. b) Analytical HPLC chromatogram profile of the subfractionation of the active component in (a). The subfractionation was done using a linear gradient of 30% to 50% B90 for 20 min at a flow rate of 1 mL/min. The active peak is indicated by the arrow. Note that the hNET active fraction is the major component of this solution. c) Analytical HPLC chromatogram profile showing the homogeneity of the active fraction in (b). The HPLC experiment was done using the same linear gradient described in (b). The component that inhibits hNET has the sequence: SVCCGYKLCFOC# (O = hydroxyproline, # = C-terminal amidation). This purified fraction is designated as χ-AuId. d) Dose-dependent inhibition of hNET by χ-AuId. IC50 value of χ-AuId against hNET is 2.6 ± 0.4 μM. This value is the average from three experiments done in triplicate. e) Comparison of the cysteine framework between χ-conotoxins and canonical T-superfamily peptides. f) Sequence logo of χ-conotoxins. Conservative substitutions in amino acids between Cys 2 and Cys 3 are observed in these peptides. The sequences are derived from Mr1.1: C. marmoreus; Bn1.5: C. bandanus; Lg1.1: C. legatus; Tx1.6: C. textile; Af1.3: C. ammiralis; Au1.4: C. aulicus; Pur1.1: C. purus; Cc1.1: C. crocatus; and Fv1.5: C. furvus.