Abstract
The evolutionarily conserved lateral habenula (LHb) enables dynamic responses to continually changing contexts and environmental conditions. A model is proposed to account for greater mnemonic and contextual control over LHb-mediated response flexibility as vertebrate brains became more complex. The medial prefrontal cortex (mPFC) provides instructions for context-specific responses to LHb, which assesses the extent to which this response information matches the motivation or internal state of the individual. LHb output either maintains a prior response (match) or leads to alternative responses (mismatch). It may also maintain current spatial and temporal processing in hippocampus (match), or alter such activity to reflect updated trajectory and sequenced information (mismatch). A response flexibility function of the LHb is consistent with poor behavioral control following its disruption (e.g., in depression).
A Core Mechanism Underlying Flexible Response Systems
A striking feature of behavioral adaptation is the rapidity with which one is able to switch learned behavioral and cognitive strategies when a goal or context changes. This switch is much faster than would be expected if use of the alternative strategy reflected new learning by different memory systems. Instead, it is generally believed that multiple memory systems must be operating in parallel (e.g., [1–3]). A challenge is to understand the mechanisms that ensure rapid, seemingly automatic, dynamic, and accurate switches in responses when the outcomes of a prior act or choice are not optimal or as expected based on memory. The ability to switch responses according to response outcomes is a fundamentally important behavior that has been attributed to the PFC [4–6]. While the PFC undoubtedly plays a role, it is worth noting that animals without a defined PFC (e.g., zebrafish [7,8]) are able to quickly and flexibly respond in adaptive ways. Therefore, one possibility is that at least an evolutionarily conserved element of the underlying neural circuitry mediating the ability to dynamically switch responses is the diencephalic epithalamus structure, the habenula (Box 1). A model presented later explains how LHb control of flexible behaviors is more context-dependent and informed by existing memories in vertebrate animals.
Box 1. Evolutionarily Conserved Habenula Integration of Sensory and Internal State Information Guides Behavioral Responses.
The habenula (derived from the Latin word habena, or ‘little rein’) is a small, elongated midline structure located on the dorsal tip of the thalamus. Especially in the earliest vertebrates, this bilateral nucleus is asymmetric in that one side is larger than the other. The right habenula nucleus of lamprey, for example, is much larger than the left habenula [99]. The habenula is also conspicuously asymmetric in fish [100], amphibians [101], and reptiles [102], but less so in birds [103] and mammals [100]. Rather than structural asymmetry, rodent and human habenula may be functionally asymmetric in that right and left habenula show differential activation [33,104]. Additional work is needed to understand the significance of such anatomical or functional asymmetries. One intriguing hypothesis [33] is that the structural (size) asymmetry may facilitate rapid and accurate control over binary behaviors such as freezing versus escaping, whereas functional lateralization may allow greater degrees of freedom in terms of information processing when controlling more complex behavioral situations and/or social interactions. In all species, however, the habenula receives sensory and internal state information, and has major projections to dopamine and serotonergic systems that are known to directly impact overt actions of animals. Thus, a common function ascribed to the habenula complex is that it serves as an integration center whereby motor output can be regulated by current sensory and internal state information [13,15,23,105,106]. Hence, habenula is considered to be essential to allow animals to switch responses as needed when environments and motivational states change.
Although the general function of the habenula may be similar across vertebrate phyla, species-specific differences in the intrinsic organization, chemistry, and connectivity patterns of its subnuclei suggest that it has continued to evolve, perhaps enabling the evolution of more complex processing by sensory, association, and motor structures, such as those in the cortex. The earliest vertebrate animals show compartmentalized habenular nuclei that reflect segregated patterns of input. Evolutionary changes in these nuclei are associated with proportional changes in output systems such as the size of efferent pathways (e.g., the fasicularis retroflexis [107]). The habenula of lamprey may contain homologues of medial and lateral divisions [99,108] that are more prominent in mammals [41,108]. However, the entire fish habenula (also described as having medial and lateral divisions) has been suggested to be homologous to only the medial habenula division of the habenular complex of reptiles and mammals [15,109]. More research is needed to understand how compartmentalizations within mammalian habenula evolved.
Complexities of the Mammalian Habenula
The mammalian habenula comprises medial and lateral divisions that can be defined by distinct gene expression profiles [9,10] and patterns of afferent and efferent connections [11,12]. As described in extensive reviews (e.g., [13–16]), the connectivity and functions of the medial habenula (MHb) or its homologs are highly conserved across vertebrate animals, receiving input from the septum, the diagonal band of Broca, and nucleus accumbens, as well as input from several sensory structures such as the pineal (vision), olfactory bulb (olfaction), and lateral line/pretectum (electrosensation). Thus internal state and sensory information comprise the bulk of MHb input. MHb efferent fibers make up a significant portion of the fasciculus retroflexus [11], a prominent and evolutionarily conserved pathway, to terminate within the interpeduncular nucleus of the midbrain [11]. MHb also projects to its neighboring LHb, and this projection does not appear to be reciprocal in nature [17].
In contrast to the MHb, the LHb is less highly conserved in terms of both the types of inputs it receives and efferent target structures. While the MHb seems to be functionally and anatomically comparable across vertebrate species, there is increasing complexity to the LHb pattern of connectivity as one moves to more complex vertebrate brains. Reflecting this complexity of connections, the LHb of mammals is often discussed as comprising medial and lateral divisions of its own [18,19]. The increasing specializations of LHb are thought to reflect broader connectivity with limbic and basal ganglia input (Figure 1) [20] as well as broader input from several structures that relay internal state information [21–23] (Figure 1). For example, in addition to input from the septum and diagonal band of Broca, the rodent LHb receives input from the entopeduncular nucleus (EPN, or globus pallidus in primates, a structure that provides emotional and motivational information [24–26]), the lateral hypothalamus, and the lateral preoptic area (LH and LPOA, involved in attention and emotional arousal, learning associations between cues and feeding behavior [27,28]), and brain areas that support a dynamic arousal system [29]. Given this prominent convergence of internal state information to the LHb, it is perhaps not surprising that the LHb appears central in mediating stress-induced changes in behavior and sensory processing [30]. Further, LHb activation is observed following exposure to stressors [31], and chronic stress leads to LHb degeneration [32].
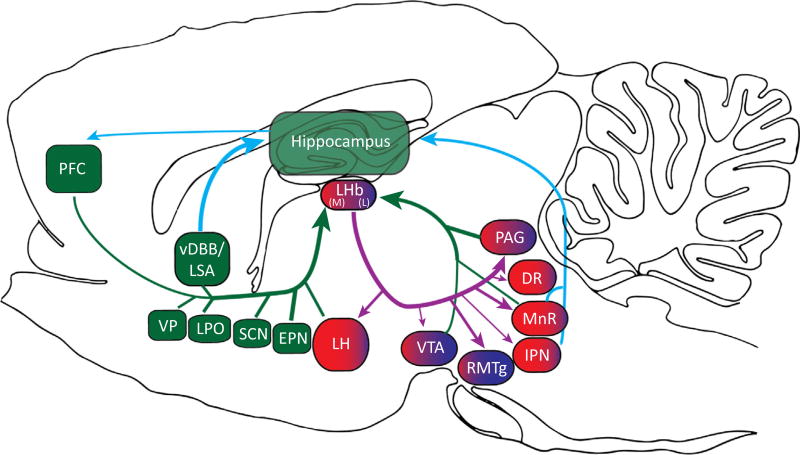
Figure 1. Illustration of the Many Afferent and Efferent Connections of the Lateral Habenula (LHb).
The structures shaded dark green (prefrontal cortex, PFC; entopeduncular nucleus, EPN; suprachiasmatic nucleus, SCN; vertical limb of the diagonal band of Broca, vDBB; and the lateral preoptic area, LPO) comprise major inputs of sensory, response, and internal state information to both medial (red) and lateral (blue) sectors of the LHb. The lateral hypothalamus (LH) also provides strong input regarding internal state and motivation, but primarily to the medial LHb. The medial and lateral LHb have overlapping and separate projections to mostly midbrain regions (ventral tegmental area, VTA; rostromedial tegmentum, RMTg; median raphe, MnR; dorsal raphe, DR; interpeduncular nucleus, IPN; and the periacqueductal grey, PAG). Of these, the MnR and IPN provide direct feedback to the hippocampus, which in turn directly connects with PFC, thereby completing the information loop across the hippocampus, PFC, and the LHb.
In sum, the habenula of all vertebrate animals may generally serve to enable flexible responding and choices in situations when sensory or internal state information change. Indeed, the vertebrate habenula has been shown to be essential for the integration of such information on a trial-by-trial basis to bias future responses (see below). As an adaptive specialization in mammals, the LHb in particular may have evolved further to integrate the more complex sensory and internal information needed to make more complex and timely choices or behavioral responses.
Approach/Avoidance Behavioral Control by the Mammalian LHb
Much of the current literature on the behavioral significance of the LHb centers on its role in processing reward and motivation information that is passed to the ventral tegmental area (VTA) and the rostromedial tegmentum (RMTg; Figure 1) to control approach and avoidance behaviors (e.g., [13,19,22,33]). Briefly, primate research suggests that the LHb passes on information that predicts aversive events and reward omission while being inhibited by encounters with unexpected rewards [34–36]. It is thought that excitatory signals from the LHb are passed on to the RMTg which in turn inhibits VTA dopamine cellular activity to promote behavioral avoidance [37]. Indeed, it has been shown that aversive stimulation activates LHb, followed by avoidance behaviors [38]. By contrast, inhibition of the LHb (e.g., during positive prediction error signaling) promotes behavioral activation. A more general interpretation of these findings could be that the LHb plays a role in determining appropriate behaviors relative to recent choice outcomes [21,23,39].
Integrated LHb signals may be passed onto midbrain regions to execute (or not) future actions via dopaminergic [40] and serotonergic [41] systems. For example, if the ongoing response or strategy of the animal results in a specific predicted and desired outcome for a given sensory and internal context, the LHb might cancel midbrain responses to the outcome [42], as is seen when sensory input accurately predicts reward outcomes [43]. However, when the ongoing response/strategy does not result in the expected outcomes, a mismatch (or prediction error) signal is generated in these midbrain areas, and this could lead to adjustments of past responses/strategies on future trials [44].
A Role for the LHb in Cortically Mediated Complex Behavior
As the cortex evolved to process more complex sensory and mnemonic information, the LHb may have co-evolved to enable the more complex sensory and memory-dependent perceptions to inform or fine-tune the ability to adaptively respond to context changes. This is supported by descriptions of the nature of cortical afferent input to the LHb in different species because those data show more intense innervation from the frontal cortex in mammals, perhaps enabling more precise and reliable behavioral control over increasingly more complex environmental situations.
Emerging functional evidence also suggests that the mammalian LHb may play a broader role in behavioral control than in non-mammalian species because it appears to be important in situations other than those needed to signal aversive events or prediction errors. Thus, a more inclusive hypothesis is that the LHb plays a crucial role in the ability of an ability to switch from the ongoing learned strategy when task or context contingencies change [21,45] (Figure 2). For example, LHb lesioned rats perform poorly on a variable (but not simple) escape platform water maze task, and this cannot be accounted for by altered attention, motivation, activity, or perseveration [46] (interestingly, the lack of LHb lesion effect on simple water maze training [30] contrasts with the significant impairment observed after RMTg lesion [47], suggesting that although the LHb and RMTg are anatomically connected [48] other inputs to these regions impact on their control over behavior). The LHb has also been shown to mediate responses in complex appetitive tasks such as probability discounting [49], as well as in spatial recognition and working memory tasks that are considered to require intact hippocampal and/or mPFC function [50,51]. Importantly, the latter deficits were not due to impaired spatial discrimination and associations per se, reward magnitude discrimination, sensory processing, or changes in motor coordination [46], suggesting that the mammalian LHb may play a key role in the general ability to change responses flexibly. This general hypothesis of LHb function is evaluated in the following sections by considering a role for the LHb in component processes that are likely involved in the ability of an animal to behaviorally adapt to changing goal conditions.
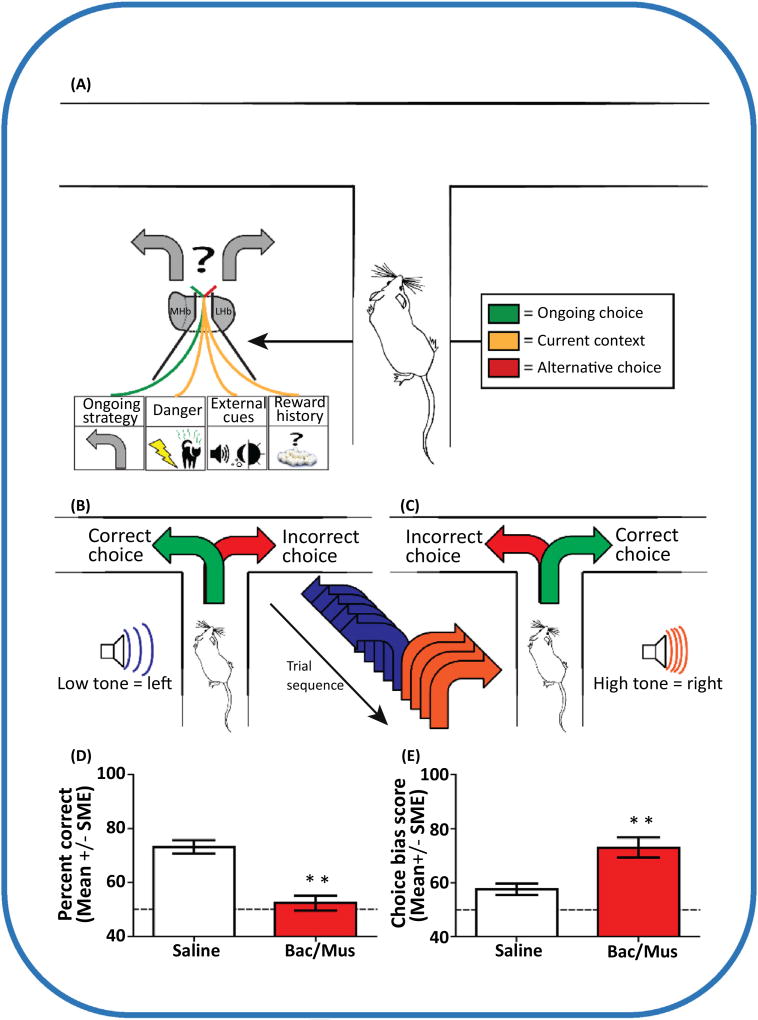
Figure 2. Maze-Based Tasks Reveal a Role for the Lateral Habenula (LHb) in Adaptive Decision Making.
(A) The LHb determines whether the ongoing strategy should continue to be employed based on currently available internal and external information, or whether the strategy should be replaced by an alternative. Current information can include the presence of danger such as the possibility of shock or predator odors, information cues such as tones or lights to guide choices, or the reward history of the ongoing strategy. In a maze-based context, many of these task conditions have been utilized to clarify the role of the LHb in adaptive decision-making [21,23,110,111]. (B) One such example is the utilization of tones to guide choice behaviors [23]. Using a trained rat, a low tone was played when the rat was in the stem arm of a T-maze, indicating that it should turn left to receive reinforcement. This tone choice pairing was repeated for three to six trials. (C) When the auditory cue was switched to a high tone, the opposite arm choice resulted in reinforcement. Over the course of a single training session a rat experienced 16 switches in tones, requiring a high level of adaptive choice behavior. (D) Inactivation of the LHb with baclofen/muscimol (Bac/Mus) resulted in chance-level performance in this task. (E) This impairment was characterized by an increased likelihood of rats to ignore the auditory cues and continue to make the same arm choice as measured by a choice-bias score (the number of errors for a single arm choice over the total number of choice errors). Data in (D,E) are adapted from [23].
LHb Processes Context- and Goal-Predictive Cue Information
Contextual information that impacts on behavioral choices reflects conditions both internal and external to an animal. The LHb is strategically located to process both types of context information. As noted earlier, the LHb receives strong input from many brain regions that process information about the current internal state in terms of motivation and emotions (e.g., EPN, LH, and POA), and changes in motivational state (e.g., increased thirst) have been shown to alter gene expression in the LHb [52]. Further, LHb lesions impair physiological and behavioral responses to chronic stress, and stress induces neural atrophy in the LHb [30,32]. In addition c-Fos expression increases in LHb after exposure to stressful conditions such as being exposed to open fields, restraint, or lithium chloride-induced illness [31]. There is emerging evidence that the LHb is exquisitely tuned to circadian cycles [53]: the LHb (and MHb) expresses intrinsic circadian changes in spontaneous firing rates [54] and receives direct input from the retina [55]. The impact of circadian rhythms on LHb function is becoming more appreciated given findings that the LHb receives input from the suprachiasmatic nucleus [56,57], and LHb neurons show higher firing rates during the light phase of the light–dark cycle of rodents, while showing greater responses to retinal stimulation during the dark phase [58].
Although the mechanism is not clear, the LHb also processes external context and goal-predictive information. Such information could reflect particular stimuli or stimulus arrays in the current sensory environment or it could reflect neural ensemble interpretations of the current sensory environment. Supporting a role for the LHb in processing specific sensory information, rodents with LHb lesions do not alter responses when the intensity of an aversive stimulus changes, and LHb neurons respond to cues that signal aversive and rewarding outcomes [10,59,60]. As evidence that LHb processes subjective interpretations of external information, LHb neurons have been observed to track choice outcome information [61]. In addition, LHb theta has been shown to co-modulate with hippocampal theta [50]. This is intriguing because of evidence that the sequencing of hippocampal neural activity relative to the past, current, or future locations of an animal preferentially occurs during theta (e.g., [62,63]) and sharp-wave ripple events (e.g., [64]) observed in the local field potentials (LFPs). Thus, although there is no known direct connection between LHb and hippocampus, communication about (expected, current, and past) spatial and temporal context information between these structures may be governed by network oscillatory activity.
The LHb Enables Flexible and Adaptive Behavioral Responses Following Changes in Learned Associations Between Context or Cues and Behaviors
The LHb appears to process a wide range of current internal and external state information that could be used to guide behavioral responses in the event that the expected outcomes are not achieved. As supporting evidence, many studies describe findings where LHb dysfunction led to an inability to change strategies and behaviors when goal, context, or sensory information changed. For example, LHb lesioned rats performed normally when required to learn a constant hidden location in a water maze, but they had difficulty learning a more complex version of the task where, on occasion, the hidden platform switched locations [46,65]. Importantly, LHb lesioned rats learned an initial strategy but were unable to learn to abandon that strategy in favor of a new one. Consistent with this pattern of effects, Flagel et al. [66] showed that c-Fos expression increased in the LHb of rats who learned a more complex version of a conditioning task, but not for rats who learned a simple discrimination, despite the fact that the overt behaviors needed to solve the tasks were the same in both groups.
Changes in behavioral strategies not only depend on the available cues or contextual information but also depend on the subjective evaluation of choice conditions such as the probability of achieving a goal or the effort/cost required to attain a goal. These evaluations are considered subjective because there is no explicitly correct or incorrect choice. Instead, choices reflect subjective preferences (e.g., level of risk tolerance). Evidence is emerging that implicates the LHb as a key player in response-switching based on the subjective evaluation of future choices. For example, LHb inactivation eliminates delay-discounting behavior [49]. That is, it disrupts flexible responding that animals need to behaviorally adjust to changing delay conditions. These LHb effects could not be attributed to changes in reward magnitude discrimination or delay discrimination.
Another type of complex subjective decision-making occurs in a probabilistic reversal task where rats first learn that location A is associated with high-probability reward, and location B with a low-probability reward. After the rat learns this discrimination, the locations of the high- and low-probability rewards are switched. After the rat learns the new locations for high- and low-probability rewards, the locations are switched back to the original locations. Rats eventually demonstrate a switch back to the original preferences, and, when they do, the locations are again reversed. The number of successful reversals within 200 trials was significantly lower for LHb inactivated rats relative to controls [21]. Without a proper LHb, rats also took longer to reach criterion after each reversal. The impaired performance on the repeated probabilistic reversal task was not due to increased perseveration per se, or to an inability to discriminate and remember associations between reward probabilities and locations [21–23]. This LHb inactivation-induced impairment parallels neurophysiological findings showing that LHb neurons respond to changed probabilities in a primate saccade-based probabilistic reversal task [39]. Together, these data suggest that the LHb is particularly important for implementing response changes when animals must track a context-dependent learned strategy or choice pattern as it changes over time. In fact, win–stay, lose–shift analyses have shown that LHb is crucial for monitoring behaviors and outcomes on a trial-by-trial basis [21,39].
LHb Mediation of mPFC Response Selection
Like other complex processes, a network of structures probably cooperate to mediate timely and specific responses. That is, the LHb is likely an important part of a response implementation network, one that includes the mPFC because it is considered to be essential for our ability to select appropriate responses when a current strategy must be overridden in favor of a new strategy for achieving a goal [5,6,67]. How the mPFC accomplishes and implements response or strategy switches is not known. Of note, however, it was recently reported that neural representational changes in mPFC occur before overt behavioral changes, suggesting that mPFC efferent structures may gate mPFC signals that a change in strategy is needed [68]. We suggest that the LHb may integrate such mPFC signals with internal state information to ultimately implement (or not) mPFC instructions.
Insight into how mPFC–LHb communication operates can be gained by studying patterns of task-relevant communication between mPFC and other connected regions such as the hippocampus and striatum [68,69] (Box 2). For example, mPFC theta comodulates with hippocampal or striatal theta, and this comodulation is related to decision-making phases of task performance and choice accuracy. In the case of hippocampus, context-specific information may be passed to mPFC because hippocampal theta leads theta recorded in mPFC [70]. mPFC theta comodulation with striatal theta seems to be important for optimal working memory functions [71,72]. Both forms of comodulation vary in strength and impact depending on current task demands and outcomes. These variations in comodulation can be reasonably expected to translate into phasic task-dependent coordination of patterns of excitation and inhibition between mPFC and efferent targets, such as the LHb, to effect behavioral changes needed for desired goal outcomes [44,73–76]. Indeed, through an elegant series of disconnection studies, Mathis et al. [77] showed that intact connections between the mPFC and the LHb are essential for normal execution of adaptive delay-discounting responses.
Box 2. Correlated Neural Activity across the Hippocampus, Prefrontal Cortex, and Lateral Habenula.
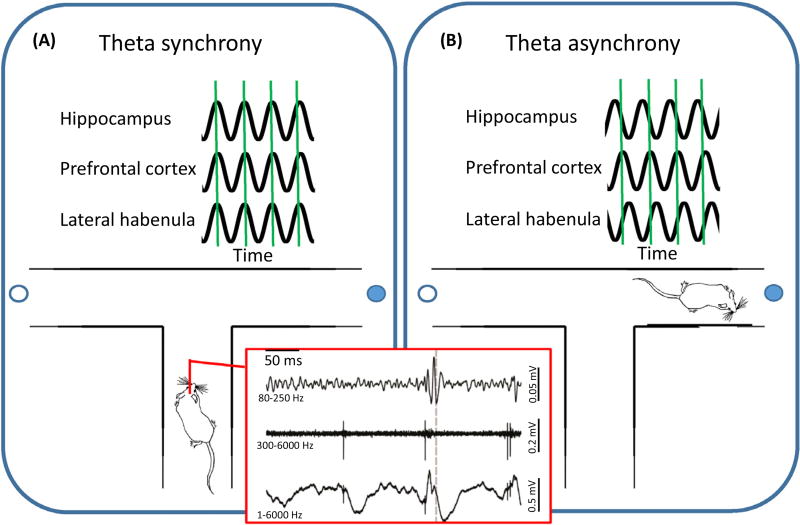
Complex behavioral functions likely rely on the integration of information across distal brain areas. Communication between connected areas is thought to be reflected in coordinated neural activity. Attempts to identify such coordinated activity include recording simultaneously from the brain areas of interest at the single cell (red box, middle trace) or population levels (red box, top and bottom traces). The most consistently reported coordinated activity has been at the population level, in particular with regard to coordinated waves of activity at the theta frequency (6–10 Hz). The left panel (Figure 1A) exemplifies such synchronized theta as a rat approaches a choice point on a maze. Such phase-locked theta rhythms have been reported to occur between the hippocampus and prefrontal cortex, and between the hippocampus and lateral habenula, when making a decision [e.g., 50,70]. Given the pattern of anatomical and functional connectivity described in the literature (see text for more details), it is predicted that the theta rhythm of the lateral habenula is also phase locked to the prefrontal theta that occurs when deciding between choices on the maze. Such coordinated activity may temporally orchestrate periods of neural plasticity across a network of brain structures to enable flexible and adaptive behavioral responses. By contrast, when animals do not need to make behavioral choices, shown in the right panel (Figure 1B), theta rhythms across the hippocampus, prefrontal cortex, and lateral habenula do not occur in synchrony (Figure I).
Figure I.
XXX.
The mPFC directly and prominently innervates LHb neurons. Notably, mPFC–LHb projecting neurons preferentially innervate the medial portion of the LHb (LHb-m), and this dominates over other cortical inputs to this region [78]. This selective innervation suggests that the mPFC–LHb projection serves to preferentially influence serotoninergic brain regions because the LHb-m strongly projects to the serotonergic areas of the median and dorsal raphe. By contrast, the lateral sector of LHb (LHb-l) receives largely basal ganglia input and has more extensive efferent connections with the dopaminergic rather than serotonergic system [10].
LHb Mediation of Flexible Responding During Goal-Directed Navigation
Given evidence linking mPFC and LHb for the purpose of enabling adaptive behavioral responses, it is of interest to consider a possible role for the LHb in flexible decisions and responses within a natural foraging context, in other words spatially extended environment for rats. This type of environmental situation engages not only limbic but also several basal ganglia structures (e.g., [79–85]). In addition, there is growing evidence for a functional link between LHb neural activity and adaptive execution of hippocampus-mediated learning and memory. As noted earlier, LHb lesions produce deficits in the hippocampus-dependent spatial memory water maze [46] and in object recognition tasks [65]. An additional study observed task-dependent theta and gamma coherence between hippocampus and LHb, and the degree of theta coherence was related to the degree of object recognition [50]. LHb inactivation was later found to disrupt spatial working memory, as demonstrated in a spatial delayed nonmatch-to-place task, even though LHb inactivation did not affect simple spatial discrimination [77]. Because there is no direct projection from the hippocampus to the LHb, mPFC may play a pivotal role in mediating information flow from hippocampus to LHb because hippocampus projects to the general area of the PFC that in turn projects to the LHb [78,86]. Although it has been shown that hippocampal leads theta comodulation with mPFC [70,87] (Box 2), it is not known whether comodulation between mPFC and LHb theta exists, and if it does, whether it is directional. The latter remains to be tested, but this is predicted because information exchange between mPFC and the LHb is essential for flexible choice behaviors [49]. Presently, however, it seems clear that the LHb plays an important role in spatial tasks that engage the hippocampus, especially those that require flexible behavioral choices.
A Proposed Model for a Limbic–LHb Response System
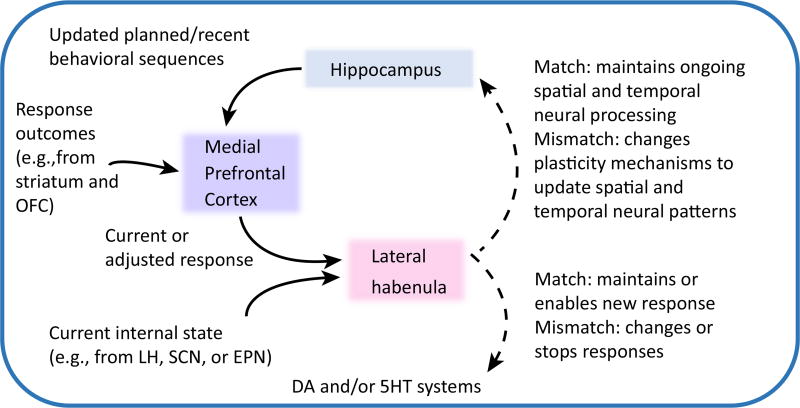
Based on the literature presented, Figure 3 presents a working hypothesis for a role the LHb may play within a broader hippocampus–mPFC–LHb circuit that allows behavioral adaptation to changing contextual (external and internal) information, such as when task contingencies change during goal-direction navigation. According to this model, hippocampus sends mPFC information about impending/recent behavioral sequences, presumably during periods of theta comodulation and/or slow-wave rhythm events. Information about the expected and actual goal outcomes of the most recent responses are relayed to mPFC via orbital frontal cortex (OFC) [88] and/or striatum [89]. Working memory capacities of mPFC enable its determination of whether expected goals were achieved given the prior response. mPFC signals efferent structures including the LHb as to whether the current response should continue or be adjusted to optimize future goal acquisition. LHb integrates mPFC input with internal state information, for example from the entopeduncular nucleus (EPN), to determine whether mPFC response-related instructions are still relevant to the current internal state or motivation. If an outcome occurs as expected for the response made, and if the internal signals indicate continued motivation to seek the goal, a ‘match’ signal from LHb would enable continuation of recent responses. However, when mPFC signals to maintain current strategic responses conflict with internal state signals (e.g., of low motivation or high stress), LHb could effectively disable mPFC instructions, resulting in reduced responding. If mPFC signals that response adjustments are needed (perhaps because goal expectations were not met) and motivation signals remain strong, LHb output would lead to the implementation of the adjusted response via dopaminergic and serotonergic activation.
Figure 3. A Working Hypothesis Describing the Hippocampus, Medial Prefrontal Cortex (mPFC), and Lateral Habenula (LHb) as a Core Neural Circuit That Enables Animals To Respond Adaptively as Task Conditions and the External and Internal Contexts Change.
The mPFC integrates information about recent action sequences and response outcomes to determine whether the current strategy or responses should continue. If the response outcomes are not as expected, the next response may need to be adjusted to achieve a desired goal. Instructions about whether to stay the course or adjust the response are fed forward to the LHb, which ultimately maintains a current response or enables a changed response depending on the extent to which information about current internal state (e.g., motivation or stress levels) warrants a behavioral switch. For example, an animal may have knowledge of what behaviors are necessary to achieve a goal such as food, but the degree of hunger or level of stress may determine whether the optimal choice is worth the perceived risks. LHb indirect (dashed line) innervation of dopaminergic and/or serotonergic systems could bias responses to stay the course or engage new behaviors. The LHb may also (via indirect connections; dashed line) inform hippocampus of the extent to which responses, recent outcomes, and internal state information occur as expected (i.e., match), which in turn can help to define the current and expected context for subsequent mnemonic processing. Abbreviations: DA, dopamine; EPN, entopeduncular nucleus; 5HT, serotonin; OFC, orbitofrontal cortex; SCN, suprachiasmatic nucleus.
LHb feedback to hippocampus is at least disynaptic via the raphe [41] or the interpeduncular nucleus [90]. Through this circuitry, LHb signals of a ‘match’ between mPFC instructions and internal state conditions could inform hippocampus that the current spatial and temporal context processing should continue. In the case of a ‘mismatch’ between the identification of optimal responses by mPFC and subcortical internal state signals, LHb messages to hippocampus may enhance plasticity mechanisms such that spatially and temporally sequenced information can be modified to reflect altered experience-dependent trajectories or responses. The degree of alteration is expected to coincide with the degree of LHb-identified mismatch because the extent and type of hippocampal place-field reorganization reflects the degree of context change (e.g., [91]). For instance, when only a subset of context features are altered, only some place fields may change (reflecting changed context information) while others do not (reflecting constant context features). If the entire context is altered, then more place fields reorganize.
The proposed loop of information flow between LHb, hippocampus, and mPFC undoubtedly reflects a simplified version of actual neural circuit functions because each of these structures continuously receives changing information of different types (e.g., sensory, memory, movement, and/or decision information). As intrinsic neurocomputations are updated with new input, the output message of each structure should reflect this dynamically varying information processing over time. In this way, the three structures in our model are expected to continually contribute unique information to the broader neural circuit underlying behavioral flexibility as long as animals continue to make experience-dependent choices in their ever-changing environments.
Concluding Remarks
There is an increasing urgency to solve the question of the cognitive and behavioral significance of the LHb because there is growing evidence that LHb dysfunction is related to disorders in which a person is not able to switch behaviors adaptively despite negative outcomes, as in the case of depression or addiction [10,92]. For example, cocaine exposure has been shown to degrade both the LHb as well as the fasciculus retroflexus, it primary efferent pathway [92]. Given the obvious role that response flexibility plays in demonstrating learned behaviors, a better understanding of LHb functions will also likely inform future therapies for memory disorders. Thus, investigation of our model should reveal new insights into a fundamental network that, when dysfunctional, can contribute to a wide range of clinical disorders characterized by poor control of behaviors.
Trends.
The complex function of the habenular complex is highly conserved across vertebrate evolution to enable flexible responding (on a trial by trial basis) in the face of changing goal expectations and internal states.
Interactions between mPFC, hippocampus, and LHb are essential for flexible and adaptive behavior during complex, but not simple, task performance.
Hippocampal theta synchrony with PFC and LHb occurs during hippocampus- and PFC-dependent decision-making.
A model is proposed that accounts for the relative contributions of the hippocampus, PFC, and LHb to the response biases of the animal. It incorporates the extent to which context-dependent goal and response expectations of an individual match their current internal state (e.g., of motivation, emotion, or biological rhythm).
Outstanding Questions.
Several challenges stand in the way of a greater understanding of how the LHb accomplishes the integration of sensory, mnemonic, motivation, and movement information to guide future responding. In addition to continuing research on the role of the LHb in basal ganglia-mediated responses, future research should focus on the topics listed below.
Identifying specific details of the sources and combinatorial patterns of extrinsic cortical connections with LHb, clearer identification of the intrinsic connectivity patterns of the LHb, as well as connectivity with the MHb, and discovery of the neural signatures and functions of the heterogeneous collection of LHb neurons. What we know so far about these issues is intriguing. For example, relative to the MHb, LHb neurons contain more elaborate dendritic arborization with postsynaptic spines [93,94], indicating that its neurons integrate more diverse information than MHb neurons. In addition, it has been reported that morphologic heterogeneity is not obviously related to the electrophysiological properties of the LHb neurons [93], such as the post-inhibitory rebound excitatory burst firing reported for LHb neurons [95]. This is surprising, but suggests that there is strong afferent control in determining neural firing patterns.
The inclusion of behavioral tasks that allow more direct comparisons across laboratories and species, and tasks that allow direct comparison of basal ganglia and limbic contributions to LHb-mediated responding. Together, these types of new information and approaches will assist efforts to understand the nature of LHb neural computations, which in turn will shed new light on the contribution of the LHb to fundamental behavioral capacities such as the ability to respond adaptively to changed situations.
Understanding how the proposed LHb function might impact on other proposed models of behavioral flexibility that include the medial prefrontal and orbital frontal cortex, as well as the amygdala and striatum. For example, there is an extensive literature that demonstrates the necessity of frontal cortical regions for behavioral flexibility [4–6,67,96–98]. The inclusion of the LHb within a broader understanding of the neural mechanisms of behavioral flexibility should expand our understanding of the important components of this process. For instance, in contrast to many frontal cortical models, the existing literature suggests that the LHb is particularly important for the type of behavioral flexibility based on trial-by-trial evaluation of goal expectations and internal states. Such results indicate that behavioral flexibility functions are scalable.
The role of the LHb in translating memory into action. If it is found with further study that a key function of the LHb is indeed to interpret and then implement cortical signals indicating that a change in response is needed, a longstanding and unresolved issue in memory research may benefit. While it has been clear for some time that different brain areas specialize in computations specific for particular types of learning and memory, it has not been clear how such mnemonic processing is translated into actions. Perhaps an underappreciated construct is that implementation of a memory or decision requires a determination that the memory or decision is consistent with the motivational or emotional state of an individual before an action occurs. The LHb may play this essential role.
Acknowledgments
Our work was supported by the National Institutes of Health (NIH) National Institute for Neurological Disease and Stroke (NINDS; grant NS094094), National Institute of Mental Health (NIMH; grant MH58755), and University Washington Royalty Research Funds (S.J.Y.M.), as well as an NIH National Institute on Drug Abuse T32 (grant DA07278, P.M.B.).
Footnotes
Supplemental Information
Supplemental information associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.tins.2017.06.001.
References
- 1.Mizumori SJY, et al. Parallel processing across neural systems: implications for a multiple memory systems hypothesis. Neurobiol. Learn. Mem. 2004;82:278–298. doi: 10.1016/j.nlm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 2.White NM, et al. Dissociation of memory systems: the story unfolds. Behav. Neurosci. 2013;127:813–834. doi: 10.1037/a0034859. [DOI] [PubMed] [Google Scholar]
- 3.Hasson U, et al. Hierarchical process memory: memory as an integral component of information processing. Trends Cogn. Sci. 2015;19:304–313. doi: 10.1016/j.tics.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalley JW, et al. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci. Biobehav. Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann. N. Y. Acad. Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- 6.Baker PM, Ragozzino ME. Contralateral disconnection of the rat prelimbic cortex and dorsomedial striatum impairs cue-guided behavioral switching. Learn. Mem. 2014;21:368–379. doi: 10.1101/lm.034819.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker MO, et al. Discrimination reversal and attentional sets in zebrafish (Danio rerio) Behav. Brain Res. 2012;232:264–268. doi: 10.1016/j.bbr.2012.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Randlett O, et al. Whole-brain activity mapping onto a zebrafish brain atlas. Nat. Methods. 2015;12:1039–1046. doi: 10.1038/nmeth.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aizawa H, et al. Molecular characterization of the subnuclei in rat habenula. J. Comp. Neurol. 2012;520:4051–4066. doi: 10.1002/cne.23167. [DOI] [PubMed] [Google Scholar]
- 10.Proulx CD, et al. Reward processing by the lateral habenula in normal and depressive behaviors. Nat. Neurosci. 2014;17:1146–1152. doi: 10.1038/nn.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herkenham M, Nauta WJH. Efferent connections of the habenular nuclei in the rat. J. Comp. Neurol. 1979;187:19–48. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- 12.Ichijo H, Toyama T. Axons from the medial habenular nucleus are topographically sorted in the fasciculus retroflexus. Anat. Sci. Int. 2015;90:229–234. doi: 10.1007/s12565-014-0252-z. [DOI] [PubMed] [Google Scholar]
- 13.Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci. Biobehav. Rev. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- 14.Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neurosci. Biobehav. Rev. 2007;31:658–672. doi: 10.1016/j.neubiorev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Bianco IH, Wilson SW. The habenular nuclei, a conserved asymmetric relay station in the vertebrate brain. Phil. Trans. R. Soc. Lond. B. 2009;364:1005–1020. doi: 10.1098/rstb.2008.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viswanath H, et al. The medial habenula: still neglected. Front. Hum. Neurosci. 2014;7:931. doi: 10.3389/fnhum.2013.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim U, Chang SY. Dendritic morphology, local circuitry, and intrinsic electrophysiology of neurons in the rat medial and lateral habenular nuclei of the epithalamus. J. Comp. Neurol. 2005;483:236–250. doi: 10.1002/cne.20410. [DOI] [PubMed] [Google Scholar]
- 18.Andres KH, et al. Subnuclear organization of the rat habenular complexes. J. Comp. Neurol. 1999;407:130–150. doi: 10.1002/(sici)1096-9861(19990428)407:1<130::aid-cne10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat. Rev. Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diaz E, et al. Morphologic and immunohistochemical organization of the human habenular complex. J. Comp. Neurol. 2011;519:3727–3747. doi: 10.1002/cne.22687. [DOI] [PubMed] [Google Scholar]
- 21.Baker PM, et al. Ongoing behavioral state information signaled in the lateral habenula guides choice flexibility in freely moving rats. Front. Behav. Neurosci. 2015;9:295. doi: 10.3389/fnbeh.2015.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker PM, et al. The lateral habenula circuitry: reward processing and cognitive control. J. Neurosci. 2016;36:11482– 11488. doi: 10.1523/JNEUROSCI.2350-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker PM, et al. Lateral habenula integration of proactive and retroactive information mediates behavioral flexibility. Neuroscience. 2017;345:89–98. doi: 10.1016/j.neuroscience.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Chavez-Martinez ME, et al. Lever pressing and active avoidance conditioning after electrolytic lesions of the entopeduncular nucleus in cats. Brain Res. Bull. 1987;18:279–284. doi: 10.1016/0361-9230(87)90003-7. [DOI] [PubMed] [Google Scholar]
- 25.Margules DL. Localization of anti-punishment actions of norepinephrine and atropine in amygdala and entopeduncular nucleus of rats. Brain Res. 1971;35:177–184. doi: 10.1016/0006-8993(71)90603-2. [DOI] [PubMed] [Google Scholar]
- 26.Lutjens G, et al. Lesions of the entopeduncular nucleus in rats prevent apomorphine-induced deficient sensorimotor gating. Behav. Brain Res. 2011;220:281–287. doi: 10.1016/j.bbr.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Sohn JW, et al. Neuronal circuits that regulate feeding behavior and metabolism. Trends Neurosci. 2013;36:504–512. doi: 10.1016/j.tins.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole S, et al. Appetitive associative learning recruits a distinct network with cortical, striatal, and hypothalamic regions. Neuroscience. 2015;286:187–202. doi: 10.1016/j.neuroscience.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kosse C, Burdakov D. A unifying computational framework for stability and flexibility of arousal. Front. Syst. Neurosci. 2014;8:192. doi: 10.3389/fnsys.2014.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heldt SA, Ressler KJ. Lesions of the habenula produce stress- and dopamine-dependent alterations in prepulse inhibition and locomotion. Brain Res. 2006;1073–1074:229–239. doi: 10.1016/j.brainres.2005.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wirshafter D, et al. Dopamine agonists and stress produce different patterns of Fos-like immunoreactivity in the lateral habenula. Brain Res. 1994;633:21–26. doi: 10.1016/0006-8993(94)91517-2. [DOI] [PubMed] [Google Scholar]
- 32.Jacinto LR, et al. The habenula as a critical node in chronic stress-related anxiety. Exp. Neurol. 2016;289:46–54. doi: 10.1016/j.expneurol.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Ichijo H, et al. An evolutionary hypothesis of binary opposition in functional incompatibility about habenular asymmetry in vertebrates. Front. Neurosci. 2017;10:595. doi: 10.3389/fnins.2016.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat. Neurosci. 2009;12:77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim U, Lee T. Topography of descending projections from anterior insular and medial prefrontal regions to the lateral habenula of the epithalamus in the rat. Eur. J. Neurosci. 2012;35:1253–1269. doi: 10.1111/j.1460-9568.2012.08030.x. [DOI] [PubMed] [Google Scholar]
- 37.Hong S, et al. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J. Neurosci. 2011;31:11457–11471. doi: 10.1523/JNEUROSCI.1384-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat. Neurosci. 2012;15:1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawai T, et al. Roles of the lateral habenula and anterior cingulate cortex in negative outcome monitoring and behavioral adjustment in nonhuman primates. Neuron. 2015;88:792–804. doi: 10.1016/j.neuron.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 40.Lammel S, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quina LA, et al. Efferent pathways of the mouse lateral habenula. J. Comp. Neurol. 2015;523:32–60. doi: 10.1002/cne.23662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Redgrave P, et al. Functional properties of the basal ganglia’s re-entrant loop architecture: selection and reinforcement. Neuroscience. 2011;198:138–151. doi: 10.1016/j.neuroscience.2011.07.060. [DOI] [PubMed] [Google Scholar]
- 43.Schultz W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 44.Mizumori SJY, Tryon VL. Integrative hippocampal and decision-making neurocircuitry during goal-relevant predictions and encoding. Prog. Brain Res. 2015;219:217–242. doi: 10.1016/bs.pbr.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Thornton EW, Evans JA. The effects of lesions of the habenula nucleus on lever press behaviour during a tandem operant schedule with contrasting response requirements. Behav. Brain Res. 1984;12:327–334. doi: 10.1016/0166-4328(84)90158-x. [DOI] [PubMed] [Google Scholar]
- 46.Thornton EW, Davies C. A water-maze discrimination learning deficit in the rat following lesion of the habenula. Physiol. Behav. 1991;49:819–822. doi: 10.1016/0031-9384(91)90324-h. [DOI] [PubMed] [Google Scholar]
- 47.Jhou TC, et al. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jhou TC, et al. The mesopontine rostromedial tegmental nucleus: a structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J. Comp. Neurol. 2009;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stopper CM, Floresco SB. What’s better for me? Fundamental role for lateral habenula in promoting subjective decision biases. Nat. Neurosci. 2014;17:33–35. doi: 10.1038/nn.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goutagny R, et al. Interactions between the lateral habenula and the hippocampus: implication for spatial memory processes. Neuropsychopharmacology. 2013;38:2418–2426. doi: 10.1038/npp.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathis V, et al. Excitatory transmission to the lateral habenula is critical for encoding and retrieval of spatial memory. Neuropsychopharmacology. 2015;40:2843–2851. doi: 10.1038/npp.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L, et al. Thirst is associated with suppression of habenula output and active stress coping: is there a role for a non-canonical vasopressin-glutamate pathway? Front. Neural Circ. 2016;10:13. doi: 10.3389/fncir.2016.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salaberry NL, Mendoza J. Insights into the role of the habenular circadian clock in addiction. Front. Psychiatry. 2016;6:179. doi: 10.3389/fpsyt.2015.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou H, Rusak B. Circadian firing-rate rhythms and light responses of rat habenular nucleus neurons in vivo and in vitro. Neuroscience. 2005;132:519–528. doi: 10.1016/j.neuroscience.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 55.Hatter S, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J. Comp. Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalsbeek A, et al. SCN outputs and the hypothalamic balance of life. J. Biol. Rhythms. 2006;21:458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- 57.Zhang C, et al. Efferent projections of prokinecticin 2 expressing neurons in the mouse suprachiasmatic nucleus. PLoS One. 2009;4:e7151. doi: 10.1371/journal.pone.0007151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao H, Rusak B. Circadian firing-rate rhythms and light responses of rat habenular nucleus neurons in vivo and in vitro. Neuroscience. 2005;132:519–528. doi: 10.1016/j.neuroscience.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 59.Thornton EW, Bradbury GE. Effort and stress influence the effect of lesion of the habenula complex in one-way active avoidance learning. Physiol. Behav. 1989;45:929–935. doi: 10.1016/0031-9384(89)90217-5. [DOI] [PubMed] [Google Scholar]
- 60.Okamoto H, et al. Genetic dissection of the zebrafish habenula, a possible switching board for selection of behavioral strategy to cope with fear and anxiety. Dev. Neurobiol. 2012;72:386–394. doi: 10.1002/dneu.20913. [DOI] [PubMed] [Google Scholar]
- 61.Bromberg-Martin ES, Hikosaka O. Lateral habenula neurons signal errors in the prediction of reward information. Nat. Neurosci. 2011;14:1209–1216. doi: 10.1038/nn.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foster DJ, Wilson MA. Hippocampal theta sequences. Hippocampus. 2007;17:1093–1099. doi: 10.1002/hipo.20345. [DOI] [PubMed] [Google Scholar]
- 63.Middleton SJ, McHugh TJ. Silencing CA3 disrupts temporal coding in the CA1 ensemble. Nat. Neurosci. 2016;19:945– 951. doi: 10.1038/nn.4311. [DOI] [PubMed] [Google Scholar]
- 64.Diba K, Buzsaki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat. Neurosci. 2007;10:1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lecourtier L, et al. Habenula lesions cause impaired cognitive performance in rats: implications for schizophrenia. Eur. J. Neurosci. 2004;19:2551–2560. doi: 10.1111/j.0953-816X.2004.03356.x. [DOI] [PubMed] [Google Scholar]
- 66.Flagel SB, et al. A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions. Neuroscience. 2011;196:80–96. doi: 10.1016/j.neuroscience.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Young JJ, Shapiro ML. Double dissociation and hierarchical organization of strategy switches and reversals in the rat PFC. Behav. Neurosci. 2009;123:1028–1035. doi: 10.1037/a0016822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Powell NJ, Redish AD. Representational changes of latent strategies in rat medial prefrontal cortex precede changes in behavior. Nat. Commun. 2016;7:12830. doi: 10.1038/ncomms12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herweg NA, et al. Theta-alpha oscillations bind the hippocampus, prefrontal cortex, and striatum during recollection: evidence from simultaneous EEG–fMRI. J. Neurosci. 2016;36:3579–3587. doi: 10.1523/JNEUROSCI.3629-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jones MW, Wilson MA. Phase precession of medial prefrontal cortical activity relative to the hippocampal theta rhythm. Hippocampus. 2005;15:867–873. doi: 10.1002/hipo.20119. [DOI] [PubMed] [Google Scholar]
- 71.Levy R, et al. Differential activation of the caudate nucleus in primates performing spatial and nonspatial working memory tasks. J. Neurosci. 1997;17:3870–3882. doi: 10.1523/JNEUROSCI.17-10-03870.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fujisawa S, et al. Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nat. Neurosci. 2008;11:823–833. doi: 10.1038/nn.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Merchant H, et al. Top-down spatial categorization signal from prefrontal to posterior parietal cortex in the primate. Front. Syst. Neurosci. 2011;5:69. doi: 10.3389/fnsys.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arnsten AFT, et al. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–239. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karlsson MP, et al. Network resets in medial prefrontal cortex mark the onset of behavioral uncertainty. Science. 2012;338:135–139. doi: 10.1126/science.1226518. [DOI] [PubMed] [Google Scholar]
- 76.Mizumori SJ, Jo YS. Homeostatic regulation of memory systems and adaptive decisions. Hippocampus. 2013;23:1103–1124. doi: 10.1002/hipo.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mathis V, et al. The lateral habenula as a relay of cortical information to process working memory. Cereb. Cortex. 2016;13:1–11. doi: 10.1093/cercor/bhw316. [DOI] [PubMed] [Google Scholar]
- 78.Kim U, Lee T. Topography of descending projections from anterior insular and medial prefrontal regions to the lateral habenula of the epithalamus in the rat: prefrontohabenular projections. Eur. J. Neurosci. 2012;35:1253–1269. doi: 10.1111/j.1460-9568.2012.08030.x. [DOI] [PubMed] [Google Scholar]
- 79.O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford University Press; 1978. [Google Scholar]
- 80.Yeshenko O, et al. Context-dependent reorganization of spatial and movement representation by simultaneously recorded hippocampal and striatal neurons during performance of allocentric and egocentric tasks. Behav. Neurosci. 2004;118:751– 769. doi: 10.1037/0735-7044.118.4.751. [DOI] [PubMed] [Google Scholar]
- 81.Puryear CB, et al. Conjunctive encoding of reward and movement by ventral tegmental area neurons: contextual control during adaptive spatial navigation. Behav. Neurosci. 2010;124:234–247. doi: 10.1037/a0018865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jo YS, et al. Effects of prefrontal cortical inactivation on neural activity in the ventral tegmental area. J. Neurosci. 2013;33:8159–8171. doi: 10.1523/JNEUROSCI.0118-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pratt WE, Mizumori SJY. Neurons in the rat medial prefrontal cortex show anticipatory rate changes to predictable differential rewards in a spatial memory task. Behav. Brain Res. 2001;123:165–183. doi: 10.1016/s0166-4328(01)00204-2. [DOI] [PubMed] [Google Scholar]
- 84.Norton ABW, et al. Independent neural coding of reward and movement by pedunculopontine tegmental nucleus neurons in freely navigating rats. Eur. J. Neurosci. 2014;33:1885–1896. doi: 10.1111/j.1460-9568.2011.07649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Redila V, et al. A role for the lateral dorsal tegmentum in memory and decision neural circuitry. Neurobiol. Learn. Mem. 2015;117:93–108. doi: 10.1016/j.nlm.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vertes RP, et al. Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. J. Comp. Neurol. 2006;499:768–796. doi: 10.1002/cne.21135. [DOI] [PubMed] [Google Scholar]
- 87.O’Neill PK, et al. Theta oscillations in the medial prefrontal cortex are modulated by spatial working memory and synchronize with the hippocampus through its ventral subregion. J. Neurosci. 2013;33:14211–14224. doi: 10.1523/JNEUROSCI.2378-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wilson RC, et al. Orbitofrontal cortex as a cognitive map of task space. Neuron. 2014;81:267–279. doi: 10.1016/j.neuron.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cromwell HC, et al. Relative reward processing in primate striatum. Exp. Brain Res. 2005;162:520–525. doi: 10.1007/s00221-005-2223-z. [DOI] [PubMed] [Google Scholar]
- 90.Lima LB, et al. Afferent and efferent connections of the interpeduncular nucleus with special reference to circuits involving the habenula and raphe nuclei. J. Comp. Neurol. 2017;525:2411– 2442. doi: 10.1002/cne.24217. [DOI] [PubMed] [Google Scholar]
- 91.Leutgeb JK, et al. Progressive transformation of hippocampal neuronal representation in ‘morphed’ environments. Neuron. 2005;48:345–358. doi: 10.1016/j.neuron.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 92.Lax E, et al. Neurodegeneration of lateral habenula efferent fibers after intermittent cocaine administration: implications for deep brain stimulation. Neuropharmacology. 2013;75:246–254. doi: 10.1016/j.neuropharm.2013.06.034. [DOI] [PubMed] [Google Scholar]
- 93.Weiss T, Veh RW. Morphological and electrophysiological characteristics of neurons within identified subnuclei of the lateral habenula in rat brain slices. Neuroscience. 2011;172:74–93. doi: 10.1016/j.neuroscience.2010.10.047. [DOI] [PubMed] [Google Scholar]
- 94.Maroteaux M, Mameli M. Cocaine evokes projection-specific synaptic plasticity of lateral habenula neurons. J. Neurosci. 2012;32:12641–12646. doi: 10.1523/JNEUROSCI.2405-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim U, Chang SY. Dendritic morphology, local circuitry, and intrinsic electrophysiology of neurons in the rat medial and lateral habenular nuclei of the epithalamus. J. Comp. Neurol. 2005;483:236–250. doi: 10.1002/cne.20410. [DOI] [PubMed] [Google Scholar]
- 96.Fiuzat EC, et al. The role of orbitofrontal-amygdala interactions in updating action-outcome valuations in Macaques. J. Neurosci. 2017;37:2463–2470. doi: 10.1523/JNEUROSCI.1839-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goschke T, Bolte A. Emotional modulation of control dilemmas: the role of positive affect, reward, and dopamine in cognitive stability and flexibility. Neuropsychologia. 2014;62:403–423. doi: 10.1016/j.neuropsychologia.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 98.Schoenbaum G, et al. A new perspective on the role of the orbitofrontal cortex in adaptive behavior. Nat. Rev. Neurosci. 2009;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yanez J, Anadon R. Afferent and efferent connections of the habenula in the larval sea lamprey (Petromyzon marinus L.): an experimental study. J. Comp. Neurol. 1994;345:148–160. doi: 10.1002/cne.903450112. [DOI] [PubMed] [Google Scholar]
- 100.Concha ML, Wilson SW. Asymmetry in the epithalamus of vertebrates. J. Anat. 2001;199:63–84. doi: 10.1046/j.1469-7580.2001.19910063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guglielmotti V, Fiorino L. Nitric oxide synthase activity reveals an asymmetrical organization of the frog habenulae during development: a histochemical and cytoarchitectonic study from tadpoles to the mature Rana esculenta, with notes on the pineal complex. J. Comp. Neurol. 1999;411:441–454. doi: 10.1002/(sici)1096-9861(19990830)411:3<441::aid-cne7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 102.Engbretson GA, et al. Habenular asymmetry and the central connections of the parietal eye of the lizard. J. Comp. Neurol. 1981;198:155–165. doi: 10.1002/cne.901980113. [DOI] [PubMed] [Google Scholar]
- 103.Gurusinghe CJ, Ehrlich D. Sex-dependent structural asymmetry of the medial habenular nucleus of the chicken brain. Cell Tissue Res. 1985;240:149–152. doi: 10.1007/BF00217568. [DOI] [PubMed] [Google Scholar]
- 104.Hetu S, et al. Asymmetry in functional connectivity of the human habenula revealed by high-resolution cardiac-gated resting state imaging. Hum. Brain Mapp. 2016;27:2602–2615. doi: 10.1002/hbm.23194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hikosaka O, et al. Habenula: crossroad between the basal ganglia and the limbic system. J. Neurosci. 2008;28:11825–11829. doi: 10.1523/JNEUROSCI.3463-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Namboodiri VMK, et al. The habenula. Curr. Biol. 2016;26:R873–R877. doi: 10.1016/j.cub.2016.08.051. [DOI] [PubMed] [Google Scholar]
- 107.Adrio F, et al. Distribution of choline acetyltransferase (ChAT) immunoreactivity in the central nervous system of a chondrostean, the Siberian sturgeon (Acipenser Baeri) J. Comp. Neurol. 2000;426:602–621. doi: 10.1002/1096-9861(20001030)426:4<602::aid-cne8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 108.Stephenson-Jones M, et al. Evolutionary conservation of the habenular nuclei and their circuitry controlling the dopamine and 5-hydroxytryptophan (5-HT) systems. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E164073. doi: 10.1073/pnas.1119348109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yanez J, Anadon R. Afferent and efferent connections of the habenula in the rainbow trout (Oncorhynchus mykiss): an indocarbocyanine dye (DiI) study. J. Comp. Neurol. 1996;372:529–543. doi: 10.1002/(SICI)1096-9861(19960902)372:4<529::AID-CNE3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 110.Nielson HC, McIver AH. Cold stress and habenular lesion effects on rat behaviors. J. Appl. Physiol. 1966;21:655–660. doi: 10.1152/jappl.1966.21.2.655. [DOI] [PubMed] [Google Scholar]
- 111.Sanders D, et al. Nicotinic receptors in the habenula: importance for memory. Neuroscience. 2010;166:386–390. doi: 10.1016/j.neuroscience.2009.12.035. [DOI] [PubMed] [Google Scholar]