Abstract
Severe central nervous system infections (CNSI), including community-acquired CNSI (CA-CNSI) and healthcare-associated ventriculitis and meningitis (HAVM), present high morbidity and mortality. Intraventricular antibiotic treatment (IVT) is advisable for these infections, though evidence is limited. We retrospectively analyzed data on 27 patients who received IVT for severe CA-CNSI and HAVM over 10 years, assessing clinical and paraclinical features, such as baseline severity and functional outcome, antibiotics, microbiological and laboratory data. Comparisons were made between patients affected by CNSI and HAVM and those with favorable and unfavorable outcomes, based on the modified Rankin scale. Gram-positive organisms dominated in CA-CNSI (64%), while gram-negative organisms were more frequent in HAVM (64%). Patients received a median of 30 days of intravenous antibiotics and 11 days of IVT, with no significant difference between CA-CNSI and HAVM. IVT-associated toxicity was rare. Patients with favorable outcomes (64%) had higher initial cerebrospinal fluid- white blood cell count (CSF-WBC), that decreased more rapidly than in patients with unfavorable outcomes. CSF-WBC dynamics did not differ between CA-CNSI and HAVM patients. Rapid decline in CSF-WBC after initiation of IVT was associated with favorable outcome. Despite severe neurological impairment at admission, most survivors achieved favorable long-term outcomes.
Keywords: Healthcare-associated ventriculitis and meningitis, Community-acquired central nervous system infections, Intraventricular antibiotics, Intrathecal antibiotics, Outcome, Modified Rankin scale
Subject terms: Neuroscience, Medical research, Neurology
Background
Community-acquired central nervous system infections (CA-CNSI)—including meningitis, meningoencephalitis, encephalitis and suppurative intracranial infections (e.g. brain abscess, subdural empyema, and epidural abscess)—are mostly associated with specific underlying conditions such as otitis, sinusitis or endocarditis1,2. Hydrocephalus can complicate the most severe cases of CA-CNSI3,4, requiring the insertion of an external ventricular drain (EVD) in selected cases to monitor intracranial pressure, to drain cerebrospinal fluid (CSF) and to administer anti-infective therapy. HAVM are severe infections that may complicate surgery in the central nervous system (CNS) or may be related to the use of neurosurgical devices or drain catheters, such as EVDs5.
CA-CNSI and HAVM result in high morbidity and mortality1,2,6,7. Intraventricular treatment with anti-infectives (IVT) is advisable in life-threatening CNS infections, to achieve a concentration of drugs in CSF, that would not be achievable with systemic therapy (ST) alone6. Direct comparison between combined IVT/ ST and ST alone favored the joint delivery, leading to faster bacterial eradication, better CSF microscopy recovery and shorter hospital stay; however, these results stem from a retrospective single-center study8. To date, only one small, randomized, single center study comparing vancomycin IVT and ST has been conducted in 10 adults with staphylococcal ventriculitis post-neurosurgery9. In summary, the current evidence on the use of IVT in HAVM is mainly based on case reports or case series5,10. In case of CA-CNSI, the evidence on the use of IVT is even scarcer6,11–13.
In this study, we report our detailed experience with the administration of IVT in patients with severe CA-CNS and HAVM treated at our center in a 10-year period. We focus on indications, microbiology, toxicity, infection relapse rate, mortality, functional short- and long-term outcome.
Methods
Informed consent was obtained from all patients or their legal guardians. The study was approved by the local ethics committee (Kantonale Ethikkommission Zürich, BASEC- Nr2021-00,631) and was conducted in accordance with the ethical standards laid down in the 2013 Declaration of Helsinki for research involving human study participants.
Study population, inclusion and exclusion criteria
This retrospective study included patients who received IVT for CA-CNSI and HAVM between January 2011 and July 2023 at the Neurocritical Care Unit (NCCU) of the University Hospital of Zurich, Switzerland. Inclusion criteria were: 1) adults (≥ 18 years old), 2) admission to the NCCU; 3) administration of IVT. Exclusion criteria was patient’s written or documented oral refusal to have his or her data analyzed for research projects.
Data collection
All data were collected from the hospital’s electronic health records (KISIM-TM Cistec® Zurich, Switzerland).
CA-CNSI includes infectious meningitis, meningoencephalitis, encephalitis, and suppurative intracranial infections (e.g. brain abscess, subdural empyema, and epidural abscess), as previously defined4,14. HAVM was defined based on the guidelines of the Infectious Diseases Society of America6, adapted for patients with hemorrhagic stroke15. CNSI was considered “severe”, if the patient required NCCU admission due to hemodynamic instability and/or respiratory or neurological failure requiring intubation, and/or need of an EVD16,17.
Demographics and baseline characteristics were collected, including: sex, age, presence of comorbidities, as assessed with the Charlson Comorbidity Index (CCI)18, estimated mortality at admission based on the Simplified Acute Physiology Score (SAPS) II19, first available Glasgow Coma Scale (GCS), and GCS at NCCU-admission. Presence of seizures and/or status epilepticus at any time during the ICU-stay was noted.
In case of CA-CNSI, specific underlying conditions such as otitis, sinusitis or endocarditis were assessed. In case of HAVM, data were collected on previous surgery (neurosurgery versus others) and primary reason for the hospitalization (hemorrhagic stroke, traumatic brain injury, ischemic stroke, brain tumor, other).
The duration and dosages of IVT and ST for CA-CNSI and HAVM were listed. Data on possible toxicity associated with IVT during the NCCU stay was sought in medical reports. (e.g. hearing loss, painful radiculitis, red man syndrome, nausea, eosinophilia, intraventricular hemorrhage, epileptic seizure).
Relapse after IVT withdrawal was defined as a new positive CSF sample (culture or broad range eubacterial polymerase chain reaction (ePCR)) after CSF sterilization and the time to relapse was noted.
Regarding outcome, data on NCCU- and hospital length of stay (NCCU-LOS and H-LOS), as well as functional outcome were collected. Functional outcome was assessed with the modified Rankin Scale (mRS) at different time points (at hospital discharge, at six, twelve and 24 months) and dichotomized in favourable (mRS ≤ 3) and unfavorable(mRS ≥ 4)20,21. Missing data were considered in the analysis.
Intravenous antibiotics
In case of suspected CNS infection, ST is started as soon as possible empirically, based on the most recent guidelines for management of patients with HAVM and CA-CNSI4,6,7,14. In case of suspected CA-CNSI, ceftriaxone and-depending on the risk factors-ampicillin are empirically initiated, together with acyclovir, where appropriate. In case of suspected brain abscess, metronidazole is added.
In case of suspected HAVM, meropenem and vancomycin are started empirically. As soon as CSF microbiology is available, ST is adapted.
IVT antibiotics
IVT is administrated through the EVD following a standardized protocol based on available data7. EVDs (Bactiseal, INTEGRA LIFESCIENCES USA) are inserted by the neurosurgical team in the operating room, the emergency room or at the NCCU under sterile conditions. Silver impregnated lines (Silverline®, Spiegelberg, Germany) are used. A single shot cefuroxime (or clindamycin in case of allergies) is given intravenously if the patient is not yet under antibiotic treatment. The inserting route involves an 8 cm tunneling between the skin insertion and the borehole.
IVT antibiotics of choice are gentamicin, vancomycin, colistin, alone or in combination, based on the possible causative microorganisms and successively on the available microbiological findings. Frequency of administration and dosage of IVT is based on the daily amount of drained CSF (Table 1, supplementary 1). The duration of treatment is highly individualized, depending on the clinical condition (e.g., HAVM versus CA-CNSI) and the pathogen (e.g., a shorter course of antibiotics is often sufficient after the removal of a foreign body infected with coagulase-negative staphylococci)10.
Table 1.
Detailed data per patient (available in a separated file). Abbreviations: f (female), m (male), IVT (intraventricular), IV (intravenous), AB (antibiotics), G (Gentamycin), V (Vancomycin), C (Colistin), SAH (subarachnoid haemorrhage), aSAH (aneurysmal subarachnoid haemorrhage), sSAH (spontaneous subarachnoid haemorrhage), mRS (modified Rankin scale), TBI (traumatic brain injury); HAVM (healthcare associated ventriculitis and meningitis), CA-CNS (Community-acquired central nervous system infections), NPH (normal pressure hydrocephalus), CVI (cerebrovascular insult), PICA (posterior inferior cerebellar artery). Time to IV - AB (days) (duration in days between day of diagnosis and begin of intravenous antibiotics); Time to IVT - AB (days) (duration in days between day of diagnosis and begin intraventricular antibiotics).
Laboratory findings and CSF diagnostics
At our institution, CSF sampling in patients with an EVD is performed routinely twice a week and whenever clinically indicated, under sterile conditions. Routinely collected parameters are CSF-WBC (available within 2 h) and microbiological culture with Gram staining (available within 24 h). In case of negative Gram staining but clinical suspicion of CSF infections, ePCR is performed. CSF glucose, lactate and protein are not routinely measured in case of HAVM, condition often associated with intraventricular blood (i.e., ventricular extension of the hemorrhage), making the values less specific/suggestive for/of infection. If necessary, CSF- WBC is corrected for the number of erythrocytes (red blood cell count [RBC]) in the same specimen, using the following formula: corrected WBC = [total WBC – (1.5 × RBC)] / 1000).
Statistical analysis
Statistical analysis was performed using R, version 4.3 and MATLAB R2023a (The MathWorks Inc., Natick, Massachusetts). Significance was defined as the probability of a two-sided type 1 error being < 5% (p-value < 0.05). Descriptive statistics are reported as counts/percentages, mean ± standard deviation (SD), or as median including the interquartile range as appropriate. All continuous data were tested for normality using Shapiro–Wilk’s test. Categorical variables were compared with Pearson’s χ2 or Fisher’s exact test, continuous/ordinal variables using Student’s t-tests or Mann–Whitney U tests for parametric and non-parametric data, respectively, where appropriate. Binary variables were tested using the Chi-Square-Test.
Survival analysis was performed using the logrank test and illustrated by Kaplan–Meier plots using MatSurv (Creed et al., (2020). MatSurv: Survival analysis and visualization in MATLAB. Journal of Open Source Software, 5(46), 1830, https://doi.org/10.21105/joss.01830). To assess between-groups differences in CSF-WBC dynamics, linear mixed-effects models were applied to logarithmized CSF-WBC values as a function of time, assuming fixed effects of time and group (including an interaction term between the two) with random intercepts and slopes. In Wilkinson notation, the models can be expressed as log(CSF-WBC) ~ time*group + (1 + time|ID). Thus, the models explore the association between CSF-WBC and time from IVT initiation while accounting for variation introduced by the group (i.e. CA-CNSI vs. HAVM, and favorable outcome vs. unfavorable outcome, respectively) and controlling for non-independence of repeated CSF-WBC measures.
Results
Demographics and clinical characteristics
Overall, 27 patients (females n = 14, 52%, age 54 ± 16 years) fulfilled the inclusion criteria during the study period. Of them, 13 (mean age 48 ± 16 years; females n = 7, 54%) suffered from CA-CNSI and 14 (mean age 60 ± 13 years, females n = 7, 50%) from HAVM. Detailed patient data are shown in Table 1. Demographics, comorbidities, severity scores at admission are presented in Table 2. Patients with CA-CNSI were younger than those with HAVM (p = 0.045). Otherwise, patients with CA-CNSI and those with HAVM did not differ in sex, CCI, and SAPS II. At NCCU-admission, median GCS was 3 [3–4] as shown in Table 2. The underlying medical conditions are presented in Table 1.
Table 2.
Demographics and clinical characteristics.
| Overall | CA-CNSI | HAVM | p-Value | |
|---|---|---|---|---|
| Number | 27 | 13 | 14 | |
| Sex (n = female, (%)) | 14 (51.9) | 7 (53.8) | 7 (50.0) | 1.000 |
| Age (as years, mean (SD)) | 54.1 (15.6) | 47.9 (16.2) | 59.9 (13.1) | 0.045 |
| CCI (median [IQR]) | 2 [0.5 – 4] | 1 [0 – 5] | 1.5 [1 – 4] | 0.921 |
| SAPS II (mean (SD)) | 43.6 (15.0) | 41.5 (16.4) | 45.9 (13.7) | 0.470 |
| GCS at hospital admission (median [IQR]) | 9 [5.5 – 13] | 10 [7 – 12] | 8 [4– 13.75] | 0.507 |
| GCS at NCCU admission (median [IQR]) | 3 [3 – 4] | 3 [3 – 4] | 3 [3 – 4] | 0.705 |
| Seizures | 0.822 | |||
| None (n (%)) | 14 (51.9) | 6 (46.2) | 8 (57.1) | |
| Focal (n (%)) | 6 (22.2) | 3 (23.1) | 3 (21.4) | |
| Generalised (n (%)) | 7 (25.9) | 4 (30.8) | 3 (21.4) | |
| Status Epilepticus | 0.564 | |||
| None (n (%)) | 22 (81.5) | 10 (76.9) | 12 (85.7) | |
| Focal (n (%)) | 1 (3.7) | 1 (7.7) | 0 (0) | |
| Generalised (n (%)) | 4 (14.8) | 2 (15.4) | 2 (14.3) |
Data in brackets represent percentages. Data in square brackets represent IQR (interquartile ranges). CA-CNSI (community-acquired central nervous system infection); CCI (Charlson Comorbidity Index); GCS (Glasgow Coma Scale); HAVM (healthcare-associated ventriculitis and meningitis); NCCU (intensive care unit); SAPS II (Simplified Acute Physiology Score II). SD (standard deviation). Comparisons among patients with CA-CNSI and those with HAVM were performed.
The occurrence of seizures or status epilepticus at any time during the NCCU-stay was comparable in patients with CA-CNSI and HAVM, as shown in Table 2.
Patients with favorable (n = 9) and unfavorable outcome (n = 18) at hospital discharge did not differ in sex, age, CCI, or SAPS II. Only GCS at NCCU admission was lower in patients with unfavorable outcome (p = 0.001) (Table 2, supplementary).
Infections and antibiotics
The identified microorganisms in CSF are listed in Table 1. The infection was caused most frequently by gram-positive microorganisms (n = 12, 44%). The majority of these patients (n = 9 out of 12, 75%) had CA-CNSI. Eight patients (30%) suffered from gram-negative CNS infection and nearly all of them (n = 7 out of 8, 87.5%) had a HAVM, as shown in Table 3. Four patients (15%) presented with mixed flora, the majority of whom (n = 3 out of 4, 75%) suffered from CA-CNSI. In three patients with HAVM it was not possible to identify a microorganism, but they were still treated for HAVM due to strong clinical suspicion of infection, based on clinical, laboratory and radiological findings. The diagnosis of CA-CNSI and HAVM was mostly made by CSF culture, as listed in Table 3.
Table 3.
Infections and antibiotics.
| Overall | CA-CNSI | HAVM | p-Value | |
|---|---|---|---|---|
| Microbiology | 0.009 | |||
| Not identified* (n (%)) | 3 (11.1) | 0 (0.0) | 3 (21.4) | |
| Gram-positive (n (%)) | 12 (44.4) | 9 (69.2) | 3 (21.4) | |
| Gram-negative (n (%)) | 8 (29.6) | 1 (7.7) | 7 (50.0) | |
| Mixed flora (n (%)) | 4 (14.8) | 3 (23.1) | 1 (7.1) | |
| Microbiology diagnosis | 0.049 | |||
| By Culture (n (%)) | 21 (77.8) | 10 (76.9) | 11 (78.6) | |
| By PCR (n (%)) | 3 (11.1) | 3 (23.1) | 0 (0.0) | |
| Only increased CSF WBC (n (%)) | 3 (11.1) | 0 (0.0) | 3 (21.4) | |
| Antibiotics for IVT | 0.206 | |||
| Colistin (n (%)) | 1 (3.7) | 1 (7.7) | 0 (0.0) | |
| Gentamicin (n (%)) | 2 (7.4) | 0 (0.0) | 2 (14.3) | |
| Vancomycin (n (%)) | 9 (33.3) | 6 (46.2) | 3 (21.4) | |
| Gentamicin and Vancomycin (n (%)) | 15 (55.6) | 6 (46.2) | 9 (64.3) | |
| Duration ST(days, median [IQR]) | 30 [16 – 47.5] | 43 [25 – 48] | 19 [15.25 – 42.75] | 0.126 |
| Duration IVT (days, median [IQR]) | 11 [7.5 – 14] | 11 [8–14] | 12.5 [7.5 – 14] | 0.644 |
| Time to culture negativity days [IQR] n = 21 patients** | 4 [2–7] | 5 [3–7] | 4 [2–6] | 0.905 |
Data in brackets represent percentages. Data in square brackets represent interquartile ranges [IQR]. CA-CNSI (community-acquired central nervous system infection); HAVM (healthcare-associated ventriculitis and meningitis); CSF-WBC (cerebrospinal fluid white blood cell count); ST (systemic intravenous antibiotics); IVT (intraventricular antibiotics); PCR (polymerase chain reaction). Comparisons among patients with CA-CNSI and those with HAVM were performed. * in three patients no microorganism was identified and IVT was guided by strong clinical suspicion of HAVM. ** In 21 patients CNSI- diagnosis was made by positive culture.
IVT was started as early as possible (Table 1). Overall, patients received ST for a duration of 30 [16–47] days and IVT antibiotics for 11 [8-14] days, with no differences between patients with CA-CNSI and HAVM (p = 0.126 and p = 0.644, respectively), as shown in Table 3. IVT antibiotics are shown in Table 1. The dosages and intervals are adjusted to quantity of CSF-drainage according to our protocol (administration scheme in supplementary material) and are equalized over all individuals.
A relapse after IVT withdrawal occurred in three (11%) patients, as shown in Table 1.
Toxicity associated with IVT antibiotics
Toxicity associated with IVT was reported in few patients (n = 3, 11%). Mild and transient eosinophilia, which was attributed to IVT administration, was reported in two patients without further signs of allergy. New epileptic seizures were temporally associated with IVT administration in two patients. Seizures were treated successfully with benzodiazepine intravenously. An exanthema was documented in one patient after IVT administration. Steroids were administered once and no additional allergy related symptoms occurred. The administration of IVT was further given.
CSF-WBC
Individual CSF-WBC time courses are shown in Fig. 1, supplementary. The majority of patients had an increased CSF-WBC on the day of IVT initiation (n = 14, 87.5%, median 596/μl [69—1920], data available for 16 patients), with no difference between CA-CNSI and HAVM (p = 0.606). Nineteen patients had at least two measurements of CSF-WBC during the first five days after IVT initiation. The majority of these patients (n = 15, 78.9%) showed a decrease in CSF-WBC from the first to the last available sample within this time frame (median decrease 66/μl per day [3-370]), with no difference between CA-CNSI and HAVM (p = 0.536).
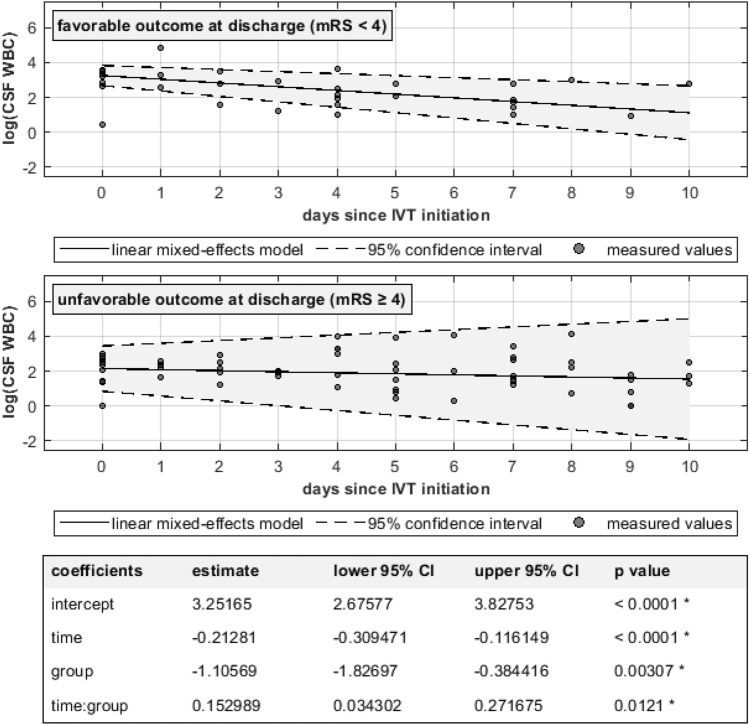
Fig. 1.
Longitudinal CSF-WBC in patients with favorable and unfavorable neurological outcome Compared to those with unfavorable outcome, patients with favorable outcome show higher CSF-WBCs at IVT initiation (p < 0.0001), but more rapid CSF-WBC decline over the course of time (p = 0.0012). Note the logarithmic y axis as a means of linearization and compact illustration of CSF-WBC over a wide range of values.
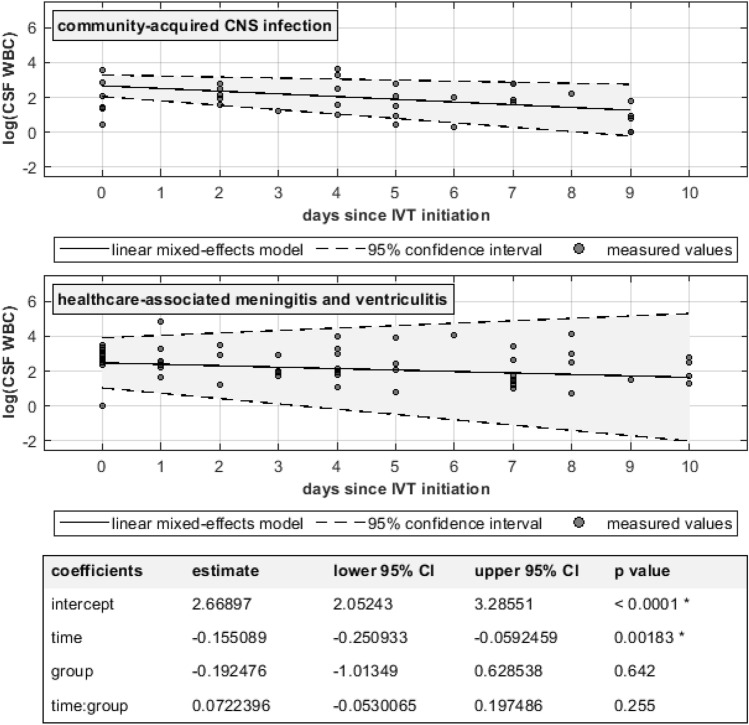
In patients with favorable outcome at hospital discharge (n = 9), CSF-WBC before IVT was higher but decreased more rapidly than in patients with unfavorable outcome, as shown in Fig. 1. On the other hand, no statistically significant difference was seen in CSF WBC dynamics between patients with CA-CNSI and those with HAVM (Fig. 2).
Fig. 2.
Longitudinal CSF-WBC in patients with CA-CNSI and HAVM. No statistically significant difference was found between the two groups. Note the logarithmic y axis as a means of linearization and compact illustration of CSF-WBC over a wide range of values.
Outcome
Overall, NCCU-LOS was 25 [16–30] days and H-LOS was 29 [23–60] days, with no differences among patients with CA-CNSI and those with HAVM (p = 0.369 and p = 0.331 respectively).
Similarly, no difference was found between CA-CNSI and HAVM with regard to long-term survival (Fig. 2, supplementary).
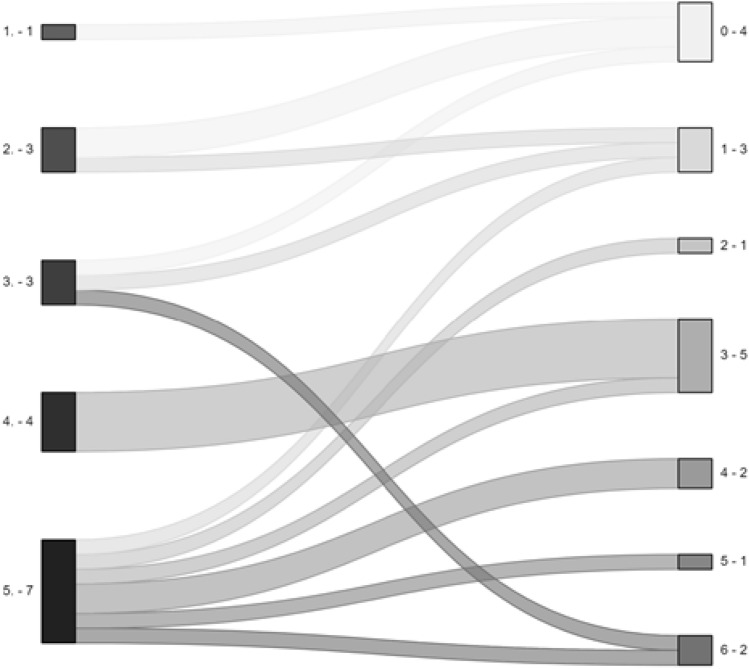
Data on functional outcome based on mRS at different time points are shown in Table 4. Five patients (18%) died during the hospital stay: three of them due to redirection of care to palliation, one due to mesencephalic bleeding and one due to aspiration pneumonia with hypoxia. Most of the survivors (16/18, 89%) improved their mRS over time (from discharge to last available follow-up, up to 24 months), as shown in Fig. 3 and Table 1.
Table 4.
Outcomes.
| Overall | CA-CNSI | HAVM | p-Value | |
|---|---|---|---|---|
| NCCU-LOS (days, median [IQR]) | 25 [16 – 30] | 22 [6 – 30] | 25.5 [19.25 – 30] | 0.369 |
| H-LOS (days, median [IQR]) | 29 [23– 60.5] | 26 [23 – 48] | 40 [27.25 – 61.75] | 0.331 |
| mRS at discharge | 0.525 | |||
| No symptoms (n (%)) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| No significant disability (n (%)) | 1 (3.7) | 1 (7.7) | 0 (0.0) | |
| Slight disability (n (%)) | 4 (14.8) | 3 (23.1) | 1 (7.1) | |
| Moderate disability (n (%)) | 4 (14.8) | 2 (15.4) | 2 (14.3) | |
| Moderate-severe disability (n (%)) | 4 (14.8) | 1 (7.7) | 3 (21.4) | |
| Severe disability (n (%)) | 9 (33.3) | 3 (23.1) | 6 (42.9) | |
| Death (n (%)) | 5 (18.5) | 3 (23.1) | 2 (14.3) | |
| mRS at 6 months | 0.729 | |||
| No symptoms (n (%)) | 3 (16.7) | 2 (20.0) | 1 (12.5) | |
| No significant disability (n (%)) | 1 (5.6) | 1 (10.0) | 0 (0.0) | |
| Slight disability (n (%)) | 4 (22.2) | 3 (30.0) | 1 (12.5) | |
| Moderate disability (n (%)) | 4 (22.2) | 2 (20.0) | 2 (25.0) | |
| Moderate-severe disability (n (%)) | 3 (16.7) | 1 (10.0) | 2 (25.0) | |
| Severe disability (n (%)) | 3 (16.7) | 1 (10.0) | 2 (25.0) | |
| Death (n (%)) | 0 (0.0) | 0 (0.00) | 0 (0.0) | |
| mRS at 12 months | 0.657 | |||
| No symptoms (n (%)) | 3 (18.8) | 2 (22.2) | 1 (14.3) | |
| No significant disability (n (%)) | 2 (12.5) | 2 (22.2) | 0 (0.0) | |
| Slight disability (n (%)) | 2 (12.5) | 1 (11.1) | 1 (14.3) | |
| Moderate disability (n (%)) | 2 (12.5) | 1 (11.1) | 1 (14.3) | |
| Moderate-severe disability (n (%)) | 4 (25.0) | 1 (11.1) | 3 (42.9) | |
| Severe disability (n (%)) | 1 (6.2) | 1 (11.1) | 0 (0.0) | |
| Death (n (%)) | 2 (12.5) | 1 (11.1) | 1 (14.3) | |
| mRS at 24 months | 0.119 | |||
| No symptoms (n (%)) | 2 (20.0) | 2 (40.0) | 0 (0.0) | |
| No significant disability (n (%)) | 2 (20.0) | 2 (40.0) | 0 (0.0) | |
| Slight disability (n (%)) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Moderate disability (n (%)) | 3 (30.0) | 1 (20.0) | 2 (40.0) | |
| Moderate-severe disability (n (%)) | 2 (20.0) | 0 (0.0) | 2 (40.0) | |
| Severe disability (n (%)) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Death (n (%)) | 1 (10.0) | 0 (0.0) | 1 (20.0) |
CA-CNSI, community-acquired central nervous system infection; HAVM (healthcare-associated ventriculitis and meningitis); H-LOS (hospital length of stay); NCCU-LOS (neurocritical care unit length of stay); mRS (modified Rankin Scale). Comparisons among patients with CA-CNSI and those with HAVM were performed.
Fig. 3.
Alluvial plot of functional status (mRS) at discharge (left) and at last follow-up (right) for all patients. (x.- y)The first number (x.) indicates the mRS score, the second one (y) the number of patients per category.
Patients with favorable outcome at hospital discharge had a shorter NCCU- and H-LOS than patients with unfavorable outcome (15 [5-17]days vs 27 [24–38] days, p = 0.002; 23 [22-26] days vs 46 [28–66] days, p = 0.010, respectively).
Key findings are summarized in Table 5.
Table 5.
Summarized key findings.
| Overall | CA-CNSI | HAVM | p-Value | |
|---|---|---|---|---|
| Age (as years, mean (SD)) | 54.1 (15.6) | 47.9 (16.2) | 59.9 (13.1) | 0.045 |
| Microbiology | 0.009 | |||
| Not identified* (n (%)) | 3 (11.1) | 0 (0.0) | 3 (21.4) | |
| Gram-positive (n (%)) | 12 (44.4) | 9 (69.2) | 3 (21.4) | |
| Gram-negative (n (%)) | 8 (29.6) | 1 (7.7) | 7 (50.0) | |
| Mixed flora (n (%)) | 4 (14.8) | 3 (23.1) | 1 (7.1) | |
| mRS at discharge | 0.525 | |||
| No symptoms (n (%)) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| No significant disability (n (%)) | 1 (3.7) | 1 (7.7) | 0 (0.0) | |
| Slight disability (n (%)) | 4 (14.8) | 3 (23.1) | 1 (7.1) | |
| Moderate disability (n (%)) | 4 (14.8) | 2 (15.4) | 2 (14.3) | |
| Moderate-severe disability (n (%)) | 4 (14.8) | 1 (7.7) | 3 (21.4) | |
| Severe disability (n (%)) | 9 (33.3) | 3 (23.1) | 6 (42.9) | |
| Death (n (%)) | 5 (18.5) | 3 (23.1) | 2 (14.3) | |
| CSF- WBC at Diagnosis (median [25th percentile – 75th percentile]) | 682 [246 – 2′944] | 1181 [685 – 66′410] | 346 [176 – 1′819] | 0.221 |
| CSF-WBC at IVT Initiation (median [25th percentile – 75th percentile]) | 596 [69 – 1′920] | 511 [91 – 1′827] | 680 [76 – 2′659] | 0.606 |
Key findings from the comparisons between patients with community-acquired central nervous system infections (CA-CNSI) and healthcare-associated ventriculitis and meningitis (HAVM) are summarized in this table. mRS: modified Rankin scale. CSF- WBC: cerebrospinal fluid white blood cell count.
Discussion
This single center study presents the experience of severely ill-patients with CA-CNSI and HAVM, who were treated with IVT. These infections are associated with a high mortality and morbidity1,5,6, but the role of IVT is unclear.
At our institution IVT was safe and survival at hospital discharge in this selected population was 81%, with most survivors improving their functional outcome further over time. In our population the kinetics rather than the absolute CSF-WBC count seems to be associated with the outcome. The spectrum of pathogens was different between CA-CNSI and HAVM.
A previous retrospective multicenter cohort study investigated practices and outcomes of 105 patients receiving IVT antibiotics for severe CNS infections in the intensive care unit (ICU) setting22. The study showed that the administration of antibiotic IVT resulted in a rate of CSF sterilization of 88.4% with a rate of culture recurrence or persistence of 9.5%. Although the population included is relatively large, the study was conducted in several centers with different treatment approaches. This variability of treatments renders generalizability challenging.
Our results suggest that the absolute CSF-WBC does not appear to be associated with outcome, but rather its rapidity of decrease. In fact, patients with favorable outcome at hospital discharge showed higher CSF-WBCs than patients with unfavorable outcome but with a more rapid decline. This is in line with previous reports indicating that the absolute values do not correlate with the outcome23,24. In these previous retrospective studies, the relationship between dynamic changes in CSF-WBC and clinical outcomes were analyzed by the area under the receiver operating characteristic curve (AUC of ROC).
After the initiation of IVT, CSF-WBCs decreased rapidly in the vast majority of patients. We can speculate that the administration of IVT antibiotics, reaching higher drug concentrations in CSF, might accelerate the normalization of CSF- WBCs. We could then postulate that IVT might therefore have a positive effect on outcome. To date, however, no study has compared CSF-WBC dynamics in patients treated with and without IVT, as repetitive lumbar puncture in patients with CA-CNSI without a CSF drainage in place is unfeasible.
Regarding the possible toxicity associated with the IVT-administration, this was rare in our study population. Due to the retrospective nature of the study, we can only report temporal associations and not direct causality.
All patients were seriously affected at the time of NCCU-admission and had a long NCCU-stay. Approximately one-fifth of the patients died during hospitalization and this is comparable to previous studies22,25. Among survivors, the vast majority recovered with a favorable long-term outcome. This finding should encourage clinicians to continue treatment even in initially very severe cases. We did not find any other study that analyzed long-term outcome. Given the heterogeneity of the diseases grouped as HAVM and CA-CNSI, it would be anyway difficult to compare the functional outcomes of these patients between studies.
In the study population, gram-positive microorganisms were mostly responsible for CA-CNSI, and gram-negative microorganisms for HAVM. Nevertheless, several patients received broad IVT for both gram-positive and gram-negative pathogens. There are two reasons for this. Firstly, in the most severe cases—as those referred to in the study—we administered a broad spectrum antibiotic therapy as microbiological results can take several days to become available. Secondly, a narrow spectrum antibiotics can be hazardous in patients with intracerebral infections. As we previously reported, about half of the patients with cerebral empyema and abscess, whose antibiotic therapy was deescalated according to the microbiological results, showed a new progression of empyema or abscess requiring a renewed escalation of antibiotics or surgical revision26. Therefore, in our opinion, de-escalation of antibiotic therapy according to culture sensitivities should be very carefully evaluated, above all in the most severe cases15.
Our analysis has several strengths. Firstly, we provide detailed information on patient characteristics (e.g. CCI), trends in laboratory parameters, microbiological data, and various outcomes with only few missing data. Secondly, we conducted comparisons between patients with different types of severe CNS infections (i.e. HAVM vs CA-CNSI) and according to functional outcome at discharge, although the sample size is small and we can only infer associations. Thirdly, our results suggest that the use of IVT may be feasible and safe, if patients already have an EVD (e.g. to treat hydrocephalus). Thirdly, it is the first study, to our knowledge, to evaluate the functional outcome over time in this population. Our data show that, despite the severe neurological impairment on admission, a favorable outcome is still possible. Finally, we treated patients homogenously according to a protocol, thus allowing comparisons between patients.
The limitations of the study are mainly due to the single center experience, the small sample size and its retrospective design. The small sample size makes it hard to identify significant changes and therefore brings up the possibility for type II errors. One major limitation is the lack of a control group—a group of patients with severe CNS infections who did not receive IVT. This substantially limits the generalizability of the results because it does not allow to directly attribute the improvement in outcome to IVT and should be further investigated. This is due to the fact that at our institution patients with severe CNS infections requiring CSF diversion via EVD, IVT is routinely given. Patients with CA-CNSI in need of CSF drainage may represent a special subset with a severe course of infection and may not be representative of the majority of CA-CNSI patients.
Additionally, CA-CNSI and HAVM include a variety of medical conditions that could alone play a relevant role in clinical course and outcome. The impact of the underlying condition should be taken into account and be considered in the design of prospective studies. With our data we are not able to make any specific disease-related recommendations. Similarly, we are not able to analyze the data for the micro-organism responsible for the severe CNS infections and their resistance to antibiotics. Furthermore, possible toxicity of IVT was only evaluated during the NCCU-stay.
Conclusions
We report our single center experience with patients with severe CNS infections treated with a standardized protocol for antibiotic IVT. Our results suggests that a rapid decline in CSF-WBC may be associated with a positive effect on the functional outcome in this population. Most patients with CA-CNSI suffered from infections with gram positive micro-organisms, while patients with HAVM had a predominance of gram negative pathogens. Mortality in the study population was comparable with previous studies. In survivors, a favorable long-term outcome was achieved in the majority of cases, despite the severe neurological impairment at admission. Prospective clinical trials are needed to define the optimal patient population for IVT, as well as its dosing and monitoring.
Supplementary Information
Abbreviations
- CNS
Central nervous system
- HAVM
Healthcare-associated ventriculitis and meningitis
- CA-CNSI
Community acquired central nervous system infections
- CNSI
Central nervous system infections
- IVT
Intraventricular antibiotics
- ICP
Intracranial pressure
- CSF
Cerebrospinal fluid
- WBC
White blood cell count
- RBC
Red blood cell count
- CSF-WBC
CSF-white blood cell count
- EVD
External ventricular drain
- ST
Systemic therapy
- NCCU
Neurocritical care unit
- ICU
Intensive care unit
- CCI
Charlson comorbidity Index
- mRS
Modified Rankin scale
- SAPS II
Simplified Acute Physiology Score
- GCS
Glasgow Coma Scale
- IQR
Interquartile range
- SD
Standard deviation
- ePCR
Eubacterial polymerase chain reaction
- LOS
Length of stay
- H-LOS
Hospital length of stay
- NCCU-LOS
Neurocritical care unit length of stay
- AB
Antibiotic
- aSAH
Aneurysmal subarachnoid haemorrhage
- sSAH
Spontaneous subarachnoid haemorrhage
- NPH
Normal pressure hydrocephalus
- CVI
Cerebrovascular insult
- PICA
Posterior inferior cerebellar artery
Author contributions
L.A., G.B. and C.T. contributed to the study conception and design. Material preparation and data collection were performed by L.A., G.R., and S.D.E. Analysis was performed by C.T., M.B. and F.S. The first draft of the manuscript was written by L.A. and G.B. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to data contained in the datasets that has not been used for this study but are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the local ethics committee (Kantonale Ethikkommission Zürich, BASEC- Nr2021-00631).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Claudio Togni and Giovanna Brandi contributed equally to this work and they share the last-authorship.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-79556-z.
References
- 1.van de Beek, D. et al. ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clin Microbiol Infec22, S37–S62 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Brouwer, M. C., Coutinho, J. M. & van de Beek, D. Clinical characteristics and outcome of brain abscess: systematic review and meta-analysis. Neurology82, 806–813 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Kasanmoentalib, E. S., Brouwer, M. C., van der Ende, A. & van de Beek, D. Hydrocephalus in adults with community-acquired bacterial meningitis. Neurology75, 918–923 (2010). [DOI] [PubMed] [Google Scholar]
- 4.van de Beek, D., Brouwer, M. C., Koedel, U. & Wall, E. C. Community-acquired bacterial meningitis. Lancet398, 1171–1183 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Karvouniaris, M., Brotis, A., Tsiakos, K., Palli, E. & Koulenti, D. Current Perspectives on the Diagnosis and Management of Healthcare-Associated Ventriculitis and Meningitis. Infect Drug Resist15, 697–721 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tunkel, A. R. et al. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin Infect Dis64, e34–e65 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein, M. et al. German guidelines on community-acquired acute bacterial meningitis in adults. Neurol Res Pract5, 44 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkie, M. D., Hanson, M. F., Statham, P. F. & Brennan, P. M. Infections of cerebrospinal fluid diversion devices in adults: the role of intraventricular antimicrobial therapy. J Infect66, 239–246 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Pfausler, B. et al. Treatment of staphylococcal ventriculitis associated with external cerebrospinal fluid drains: a prospective randomized trial of intravenous compared with intraventricular vancomycin therapy. J Neurosurg98, 1040–1044 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Nau R, Blei C, Eiffert H. Intrathecal Antibacterial and Antifungal Therapies. Clin Microbiol Rev 2020;33. [DOI] [PMC free article] [PubMed]
- 11.Helweg-Larsen, J. et al. Pyogenic brain abscess, a 15 year survey. BMC Infect Dis12, 332 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeshita, M., Kagawa, M., Izawa, M. & Takakura, K. Current treatment strategies and factors influencing outcome in patients with bacterial brain abscess. Acta Neurochir (Wien)140, 1263–1270 (1998). [DOI] [PubMed] [Google Scholar]
- 13.Song, L. et al. Clinical features and outcome analysis of 90 cases with brain abscess in central China. Neurol Sci29, 425–430 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Bodilsen, J. et al. European society of Clinical Microbiology and Infectious Diseases guidelines on diagnosis and treatment of brain abscess in children and adults. Clin Microbiol Infect30, 66–89 (2024). [DOI] [PubMed] [Google Scholar]
- 15.Pietrzko E, Bogli S, Frick K, et al. Broad Range Eubacterial Polymerase Chain Reaction of Cerebrospinal Fluid Reduces the Time to Exclusion of and Costs Associated with Ventriculostomy-Related Infection in Hemorrhagic Stroke. Neurocrit Care 2023. [DOI] [PMC free article] [PubMed]
- 16.Glimaker, M. et al. Neuro-intensive treatment targeting intracranial hypertension improves outcome in severe bacterial meningitis: an intervention-control study. PLoS One9, e91976 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edberg, M., Furebring, M., Sjolin, J. & Enblad, P. Neurointensive care of patients with severe community-acquired meningitis. Acta Anaesthesiol Scand55, 732–739 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis40, 373–383 (1987). [DOI] [PubMed] [Google Scholar]
- 19.Le Gall, J. R., Lemeshow, S. & Saulnier, F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA270, 2957–2963 (1993). [DOI] [PubMed] [Google Scholar]
- 20.Gaastra, B. et al. Evidence-based interconversion of the Glasgow Outcome and modified Rankin scales: pitfalls and best practices. J Stroke Cerebrovasc Dis31, 106845 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Awad, E. M., Humphries, K. H., Grunau, B. E. & Christenson, J. M. Premenopausal-aged females have no neurological outcome advantage after out-of-hospital cardiac arrest: A multilevel analysis of North American populations. Resuscitation166, 58–65 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Lewin, J. J. 3rd. et al. Current Practices of Intraventricular Antibiotic Therapy in the Treatment of Meningitis and Ventriculitis: Results from a Multicenter Retrospective Cohort Study. Neurocrit Care30, 609–616 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Montes, K., Jenkinson, H., Habib, O. B., Esquenazi, Y. & Hasbun, R. Corrected white blood cell count, cell index, and validation of a clinical model for the diagnosis of health care-associated ventriculitis and meningitis in adults with intracranial hemorrhage. Clin Neurol Neurosurg178, 36–41 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Qu, J., Jiang, J. & Lv, X. The utility of cerebrospinal fluid white cell count during the prognostic assessment for cryptococcal meningitis patients: a retrospective study. BMC Infect Dis20, 571 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van de Beek, D. et al. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med351, 1849–1859 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Dietler, S. et al. Spontaneous empyema and brain abscess in an intensive care population: clinical presentation, microbiology, and factors associated with outcome. Acta Neurochir (Wien)165, 651–658 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to data contained in the datasets that has not been used for this study but are available from the corresponding author on reasonable request.