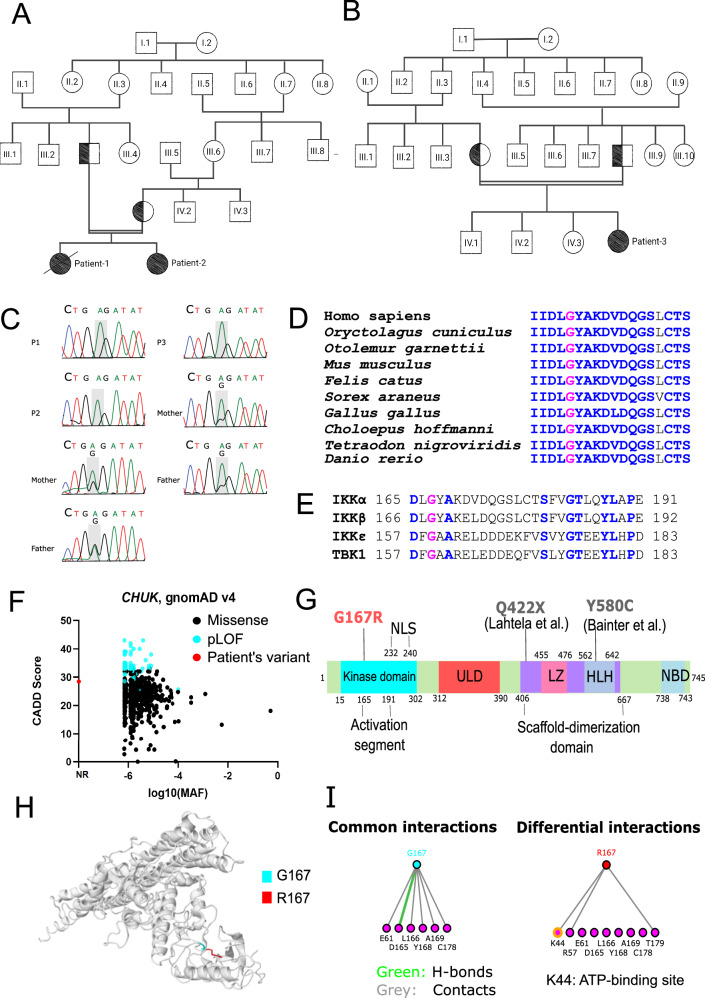
Fig. 1. Identification of IKKαG167R homozygous germline missense variant in three patients.
A, B Family pedigree of P1, P2 and P3 showing the consanguineous marriage. All parents of patients are heterozygous for this variant and germline transmission is responsible for the homozygosity. Patient-1 is deceased as indicated by the black diagonal line. Double horizontal lines in pedigree indicate consanguinity. C Sanger sequencing results of patients and their parents, showing the nucleotide change 499 G > A in the component of inhibitor of nuclear factor kappa B kinase complex (CHUK) gene coding for IKKα. D Evolutionary conservation of G167 residue in IKKα of different species is shown based on the multiple sequence alignment. G167 is indicated by pink, conserved residues are indicated by blue and non-conserved residues are indicated by black. E G167 residue in IKKα (indicated by pink) is conserved in the kinase domain of other IKK and IKK-related kinases (IKKβ, IKKε and TANK binding kinase 1 (TBK1)). Other conserved residues are indicated by blue and non-conserved residues are indicated by black. F CADD vs MAF plot of patient’s variant together with all missense and predicted loss-of-function (pLOF) CHUK gene variants (797 variants in total) obtained from the Genome Aggregation Database (gnomAD) v4 datasets. NR: Not reported. G Different domains and previously reported homozygous variants in human IKKα. Kinase domain, ULD: ubiquitin-like domain, LZ: Leucine zipper, HLH: Helix-Loop-Helix, NBD: NEMO-binding domain, NLS: Nuclear localization signal. Q422X and Y580C variants were previously reported in other studies. H The impact of IKKαG167R on previously reported structure of IKKαWT (PDB: 5EBZ) was indicated using Missense3D tool. G167 is indicated by blue and R167 is by red. I The effect of IKKαG167R variant on interactions of different residues was investigated using I see in 3D (iCn3D) Structure Viewer (PDB: 5EBZ). Common and different interactions between IKKαWT and IKKαG167R were indicated. A notable differential interaction between residue G167 and ATP-binding site K44 (yellow in colour) was indicated. Green colour indicates H-bonds and grey colour indicates contacts with residues.