Abstract
Background
Although central disorders of hypersomnolence (CDH) and attention‐deficit/hyperactivity disorder (ADHD) are frequently comorbid, they often remain underdiagnosed, leading to insufficient treatment and sociopsychological outcomes.
Case Presentation
Here, we present a case of a male in his late 20s with ADHD and autism spectrum disorder who exhibited symptoms suggestive of idiopathic hypersomnia (IH), a subtype of CDH. The patient experienced difficulty waking up and dropped out of university. Additionally, although methylphenidate extended‐release was prescribed, he often forgot to take his medication, resulting in difficulty waking up until late afternoon. No symptoms related to rapid eye movements sleep were observed. Considering the possibility of concurrent hypersomnia with neurodevelopmental disorders, we conducted 24‐h polysomnography (PSG). The results demonstrated total sleep time of 774.5 min (≥660 min). Together with other criteria, we diagnosed him as having IH. Following discharge and discussion with the patient, we provided sleep hygiene education for him, and he resumed day care attendance to establish a social routine.
Conclusion
In cases where hypersomnia may co‐occur with neurodevelopmental disorders, active utilization of 24‐h PSG enables detailed evaluation of sleep–wake patterns and behaviors, facilitating effective guidance on sleep hygiene and promoting improvements in social rhythms and sociability.
Keywords: attention‐deficit/hyperactivity disorder, autism spectrum disorder, hypersomnolence, idiopathic hypersomnia, polysomnography
A 24‐h polysomnography (PSG) is useful for diagnosing the idiopathic hypersomnia patient with neurodevelopmental disorders.
BACKGROUND
Attention‐deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterized by the inattentive and hyperactive–impulsive symptoms. 1 One of its comorbidities is excessive daytime sleepiness (EDS), which is frequently observed in clinical settings. 2 , 3 Idiopathic hypersomnia (IH) is a type of central disorder of hypersomnolence (CDH) characterized by EDS, severe sleep inertia, and prolonged nighttime sleep despite normal sleep. 4 A key issue is that it is unclear whether EDS is the cognitive and behavioral consequences of ADHD or features of IH, potentially contributing to misdiagnosis or delayed diagnosis in many patients, potentially hampering their optimized treatment and daily functioning. 5
Autism spectrum disorder (ASD) is characterized by difficulties in social communication and interaction, restricted interest, and repetitive behaviors. 1 These features are occasionally observed in individuals with ADHD in a clinical setting. Sleep disturbances are frequently observed in individuals with ASD. 6
IH is typically diagnosed by the standard criteria, including polysomnography (PSG) and multiple sleep latency testing (MSLT), 24‐h PSG or sleep–wake log and actigraphy. 4 However, considering the low sensitivity and test–retest reliabilities of the MSLT for IH and narcolepsy type 2 (NT2), a different approach will be needed in the classifications of patients who have genuine complaints of hypersomnia but fail to have diagnostic MSLT results. 7 , 8 Specifically, mean sleep latency (MSL) of 8 min or less is used clinically to determine daytime sleepiness, but abnormal MSL is frequently found in the general population. 9 , 10 In addition, the standard protocol by the American Academy of Sleep Medicine (AASM) 11 recommends that daytime video‐monitoring, not PSG, is sufficient even for suspected cases with IH, although detecting lapses of sleep between MSLT sessions could be difficult in some cases, and detailed objective evaluation of behavior is not possible through sleep–wake log and actigraphy.
Here, we report a case with ADHD and autism spectrum disorder (ASD) who presented symptoms suggestive of IH, and 24‐h PSG was applied for its diagnosis.
CASE PRESENTATION
A male in his late 20s was referred to our hospital. He exhibited delayed language development, minimal response to social cues, and difficulty establishing eye contact, as well as a tendency to easily redirecting attention and forgetting items since childhood. Due to difficulties communicating with school classmates, he became maladjusted at school and developed self‐injurious behaviors, leading to consultation at a clinic specializing in child psychiatry around the age of 10. He then received diagnoses of ADHD and ASD. Because several secondary symptoms, including withdrawal, anxiety and depressive state, were observed in addition to communication difficulties and inattention, he received both psychological education and pharmacotherapy with methylphenidate and paroxetine. On reaching adulthood, he was referred to another clinic that specialized in adult psychiatry.
At the clinic, a day care group therapy was introduced in addition to the pharmacotherapy for the improvement of his daily activity and social skills. However, his pronounced inattentive symptoms prevented him from regular visits to the clinic and medication adherence. In addition, he often slept over 12 h a day, and also his wake‐up time varied from 10 a.m. to 1 p.m., even when taking regular medication. Such sleep disturbances and EDS that he experienced significantly impacted his daily activities and regular treatment and care at the clinic. Therefore, he was referred to our hospital for detailed examination of a possible comorbid sleep‐related disorder.
At his first visit, we suspected a CDH, especially NT2 or IH due to his clinical history, a prolonged sleep time and absence of cataplexy. Regarding sleep‐breathing disorders, he showed no symptoms like snoring or nocturnal sweating. Then, we performed 24‐h PSG according to the standard guidelines. He was invited to sleep as long as possible, ad libitum. Sunlight, television, smartphone, tablet computer, watches, and visits from family or friends were forbidden during the 24‐h PSG recording. In addition, the patient refrained from caffeinated beverages for 24 hour before and during the recording. Communication with medical staff was limited to emergencies, replacing an electrode (if the need arose), and meal delivery. The sleep room was maintained in a dim light (10 lux) and 24‐h PSG was performed in psychostimulant‐free conditions with the patient's permission. PSG included electroencephalographic electrodes (F3/A2, F4/A1, C3/A2, C4/A1, O1/A2, and O2/A1), bilateral electrooculogram, submental surface electromyogram, and electrocardiography. PSG recordings were scored manually for total recording time (TRT), total sleep time (TST), sleep latency (SL), REM SL, sleep efficiency (SE), sleep stages, and arousal index (ArI) according to the standard criteria. 12
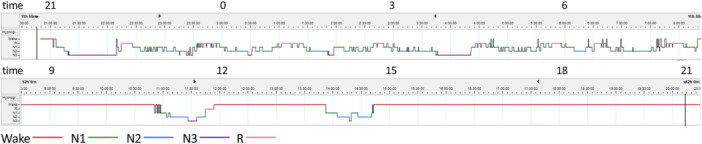
All PSG recording parameters are shown in Table 1. The 24‐h PSG recording revealed that TRT was 1417.5 min. The patient slept for 774.5 min. The hypnogram is pictured in Figure 1. He fell asleep normally (SL, 13.5 min) and almost did not experience arousals during sleep (SE, 92.8% and ArI, 15.2). The percentage of each sleep stage was normal for his age. Sleep‐onset REM period (SOREMP) was not represented. During the recording, he was observed eating udon (wheat noodles) before the evening meal on day 2, despite having already eaten his regular meals. He was diagnosed with IH according to the diagnostic criteria and clinical judgment.
Table 1.
Sleep parameters on 24‐h PSG.
| Sleep parameter | Findings |
|---|---|
| Total recording time, min | 1417.5 |
| Total sleep time, min | 774.5 |
| Sleep efficiency, % | ‐ |
| Nocturnal | 92.8 |
| Overall | 55.0 |
| Sleep latency, min | ‐ |
| Non‐REM | 13.5 |
| REM | 71.5 |
| Sleep stage N1, % | 30.4 |
| Sleep stage N2, % | 38.3 |
| Sleep stage N3, % | 14.4 |
| Sleep stage REM, % | 16.9 |
| Arousal index | ‐ |
| Nocturnal | 14.4 |
| Overall | 15.2 |
Abbreviations: PSG, polysomnography; REM, rapid eye movements.
Figure 1.
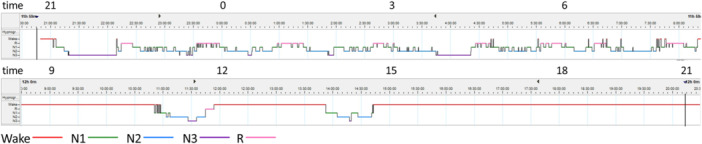
Hypnogram from a 24‐h polysomnography (PSG) showing sleep–wake status in a day. A hypnogram shows the transition from non‐rapid eye movements (non‐REM) sleep to REM sleep and its cycle.
DISCUSSION
To the best of our knowledge, this is the first reported case showing the usefulness of performing 24‐h PSG to diagnose a patient with ADHD and ASD who is comorbid with IH. The 24‐h PSG enables to record not only sleep–wake state, but also behaviors in detail.
Previous studies revealed high frequency of ADHD symptoms in patients with central hypersomnia, including IH. 13 , 14 , 15 Of 602 adults with possible ADHD, 339 (56.3%) showed EDS, 2 while 47 of 100 patients with ADHD presented EDS and 22 of them (46.8%) were diagnosed as having NT2 or IH according to the DSM‐5. 3 Although the shared underlying mechanism of ADHD and IH remains to be elucidated, some studies reported that the arousal‐dysregulation hypothesis postulated for ADHD might be an appropriate model for sleep disordered patients with EDS. 15 , 16
Most recently, a case report of ADHD similar to ours was published. 17 In the case, MSLT findings reached the IH diagnostic criteria, but 24‐h recording resulted in total sleep time of <660 min, which did not fulfill the criteria of 660 min. The patient also suffered from gaming disorder. The case highlighted educationally that video monitoring is useful for detecting a sleep attack. Compared to the reported case, our patient was diagnosed with IH by 24‐h PSG, and TST was 774.5 min. The patient had been diagnosed with both ADHD and ASD. Moreover, 24‐h PSG revealed that the patient ate udon noodles at an inadequate time. The detailed objective evaluation provided us with an opportunity to initiate comprehensive interventions focusing on improving sleep hygiene and dietary habits, tailored to the developmental characteristics of the patient. Subsequent discussions with the patient underscored the significance of this evaluation, leading to tangible improvements in his sleep patterns and the resumption of day care attendance, thereby facilitating the establishment of a social routine. The active utilization of 24‐h PSG is effective when attention‐deficit, an ADHD symptom, causes inadequate behaviors and social difficulties.
As for ASD, sleep disturbances such as insomnia, parasomnias, and circadian rhythm sleep–wake disorders are frequently reported. 6 Indeed, parent‐reported sleep problem rates for children with ASD range from 50% to 80%, compared to 9% to 50% in comparison groups. 18 The relationship between sleep disorders and developmental stages in ASD remains unclear, as both a large retrospective‐longitudinal study 19 and a 1‐year follow‐up study 20 failed to reveal a significant relationship between them. Regarding the associations between sleep disturbances and ASD symptoms, reduced sleep duration has been linked to and predicts increased ASD symptom severity, including difficulties in social skills 18 and communication, higher rates of stereotypic behaviors, and stricter adherence to nonfunctional routines. 21
A small number of PSG studies have reported on the evaluation of sleep disorders in ASD: adolescents with autism had longer sleep latency, more nocturnal awakenings, lower sleep efficiency, increased stage 1 sleep duration, decreased non‐REM and slow‐wave sleep, fewer stage 2 EEG spindles, and fewer REM sleep eye movements compared to controls. 22 Children with autism have shorter total sleep time, more slow wave sleep, less REM sleep, and longer REM sleep latency than typically developing children. 23 More non‐REM parasomnias were observed in 14 children with ASD on PSG than in the controls. 24 REM sleep behavior disorder was identified in five of 11 patients. 25 Thus, it is suggested that disturbances in sleep maintenance might be more predominant than hypersomnia in ASD. Several studies have indicated that blood levels of melatonin, which promotes sleep, 26 are decreased in individuals with autism, 27 and abnormalities in the circadian rhythm of melatonin secretion have been noted, 28 supporting the above findings.
In this case report, we have demonstrated the utility of 24‐h PSG in diagnosing a patient with comorbid ADHD, ASD, and IH. However, it should be noted that ADHD cases that have been studied until now might have ASD comorbidity. Previous research has suggested shared neural mechanisms between ADHD and ASD, 29 , 30 , 31 indicating that ASD traits, including those at a subclinical level, may be present in some individuals with ADHD. Additionally, the change in diagnostic criteria (the coexistence of ADHD and ASD was not permitted until DSM‐5) may also have an impact. Therefore, interpretation should be exercised with caution with regard to this issue.
The latest diagnostic criteria of IH is based on the presence of EDS for >3 months, absence of cataplexy, <2 SOREMPs on the MSLT or the preceding PSG, and MSL < 8 min on the MSLT, or a total 24‐h sleep time ≥660 min on 24‐h PSG or by wrist actigraphy in association with a sleep–wake log (averaged over at least 7 days with unrestricted sleep). 4 In addition, PSG and MSLT findings are not consistent with a diagnosis of narcolepsy type 1 (NT1) or NT2, which means ≥2 SOREMPs on the MSLT or the preceding PSG and MSL ≤ 8 min. 4 Other causes of hypersomnolence, including insufficient sleep, need to be ruled out. 4 However, recently, as the CDH has been considered as a spectrum of disorders except for NT1 with clear cataplexy and/or decreased orexin/hypocretin concentration in the cerebrospinal fluid, the diagnostic classification and its criteria is now in a transitional phase. 8
In clinical settings, EDS is observed in some cases of neurodevelopmental disorders. 4 , 32 , 33 For example, research has shown a link between the inattentive type of ADHD and hypersomnia, 4 , 15 indicating potentially shared underlaying mechanism. This suggests that some individuals with neurodevelopmental disorders might present clinical features resembling secondary “idiopathic hypersomnia”. However, the definition of CDH has changed over time and remains subject to interpretation. In the present case, we diagnosed IH based on the standard diagnostic criteria, 4 which stated that the symptoms and signs of the idiopathic hypersomnia are not better explained by a circadian rhythm sleep‐wake disorder or other current sleep disorders, medical disorder, mental disorder, or medication/substance use of withdrawal. Nevertheless, future research is necessary to better characterize and define the subgroup of individuals who experience both hypersomnia and neurodevelopmental disorders.
This case has two main limitation. First, it is a single case report; therefore, further studies are necessary to generalize the findings. Second, although the guidelines recommend discontinuing psychostimulants two weeks before sleep‐wake evaluation, 11 our patient discontinued them two days prior. However, this likely had a minimal impact given the drug's serum half‐life of approximately 3.5 hours. 34
CONCLUSION
This case highlights the utility of 24 h PSG in diagnosing neurodevelopmental disorders with suspected IH, and may also assist in improving sleep‐wake and social rhythms. Further research should focus on identifying subtypes of neurodevelopmental disorders and symptom dimension, particularly regarding IH comorbidity.
AUTHOR CONTRIBUTIONS
Keisuke Kido and Naoko Sugita performed the 24‐h PSG. Keisuke Kido and Manabu Kubota drafted the manuscript. Naoko Sugita, Naoko Tachibana, and Toshiya Murai critically reviewd and revised the manuscript. All authors have read and approved the final version.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS APPROVAL STATEMENT
N/A.
PATIENT CONSENT STATEMENT
Written informed consent was obtained from the patient for publication of the case report.
CLINICAL TRIAL REGISTRATION
N/A.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Naohiro Egawa (Department of Neurology, Graduate School of Medicine, Kyoto University) for managing a PSG device. The authors have no funding to report. We also would like thank Editage (www.editage.jp) for their assistance with English‐language editing.
Kido K, Sugita N, Murai T, Tachibana N, Kubota M. Diagnostic usefulness of 24‐h polysomnography for idiopathic hypersomnia co‐occurring with neurodevelopmental disorders: A case report. Psychiatry Clin Neurosci Rep. 2024;3:e70032. 10.1002/pcn5.70032
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Neurodevelopmental disorders . In: First Michael B editors. Diagnostic and statistical manual of mental disorders. 5th ed. American Psychiatric Association Publishing; 2022. p. 35–99. [Google Scholar]
- 2. Ito W, Komada Y, Okajima I, Inoue Y. Excessive daytime sleepiness in adults with possible attention deficit/hyperactivity disorder (ADHD): a web‐based cross‐sectional study. Sleep Med. 2017;32:4–9. [DOI] [PubMed] [Google Scholar]
- 3. Lopez R, Micoulaud‐Franchi JA, Camodeca L, Gachet M, Jaussent I, Dauvilliers Y. Association of inattention, hyperactivity, and hypersomnolence in two clinic‐based adult cohorts. J Atten Disord. 2020;24(4):555–564. [DOI] [PubMed] [Google Scholar]
- 4. American Academy of Sleep Medicine . International classification of sleep disorders. 3rd ed. American Academy of Sleep Medicine; 2023. [Google Scholar]
- 5. Arnulf I, Thomas R, Roy A, Dauvilliers Y. Update on the treatment of idiopathic hypersomnia: progress, challenges, and expert opinion. Sleep Med Rev. 2023;69:101766. [DOI] [PubMed] [Google Scholar]
- 6. Singh K, Zimmerman AW. Sleep in autism spectrum disorder and attention deficit hyperactivity disorder. Semin Pediatr Neurol. 2015;22(2):113–125. [DOI] [PubMed] [Google Scholar]
- 7. Evangelista E, Lopez R, Barateau L, Chenini S, Bosco A, Jaussent I, et al. Alternative diagnostic criteria for idiopathic hypersomnia: a 32‐hour protocol. Ann Neurol. 2018;83(2):235–247. [DOI] [PubMed] [Google Scholar]
- 8. Lammers GJ, Bassetti CLA, Dolenc‐Groselj L, Jennum PJ, Kallweit U, Khatami R, et al. Diagnosis of central disorders of hypersomnolence: a reappraisal by European experts. Sleep Med Rev. 2020;52:101306. [DOI] [PubMed] [Google Scholar]
- 9. Mignot E. Correlates of sleep‐onset REM periods during the multiple sleep latency test in community adults. Brain. 2006;129(6):1609–1623. [DOI] [PubMed] [Google Scholar]
- 10. Singh M, Drake CL, Roth T. The prevalence of multiple sleep‐onset rem periods in a population‐based sample. Sleep. 2006;29(7):890–895. [DOI] [PubMed] [Google Scholar]
- 11. Krahn LE, Arand DL, Avidan AY, Davila DG, DeBassio WA, Ruoff CM, et al. Recommended protocols for the Multiple Sleep Latency Test and Maintenance of Wakefulness Test in adults: guidance from the American Academy of Sleep Medicine. J Clin Sleep Med. 2021;17(12):2489–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events v2.6 (Print). American Academy of Sleep Medicine; 2020. [Google Scholar]
- 13. Vernet C, Leu‐Semenescu S, Buzare MA, Arnulf I. Subjective symptoms in idiopathic hypersomnia: beyond excessive sleepiness: subjective symptoms in idiopathic hypersomnia. J Sleep Res. 2010;19(4):525–534. [DOI] [PubMed] [Google Scholar]
- 14. Bayard S, Croisier Langenier M, Cochen De Cock V, Scholz S, Dauvilliers Y Executive control of attention in narcolepsy. Yung Weditor. PLoS ONE. 2012;7(4):e33525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dodson C, Spruyt K, Considine C, Thompson E, Ipsiroglu OS, Bagai K, et al. Hyperactivity in patients with narcolepsy and idiopathic hypersomnia: an exploratory study. Sleep Sci Pract. 2023;7(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Owens J, Gruber R, Brown T, Corkum P, Cortese S, O'Brien L, et al. Future research directions in sleep and ADHD: report of a consensus working group. J Atten Disord. 2013;17(7):550–564. [DOI] [PubMed] [Google Scholar]
- 17. Asaga T, Hashimoto K, Kawamura Y, Fujita N, Kimata M, Sekizawa A, et al. Idiopathic hypersomnia with a video recording of a spontaneous sleep attack: a case report. Medicine. 2024;103(7):e36782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Richdale AL, Schreck KA. Sleep problems in autism spectrum disorders: prevalence, nature, & possible biopsychosocial aetiologies. Sleep Med Rev. 2009;13(6):403–411. [DOI] [PubMed] [Google Scholar]
- 19. Mayes SD, Calhoun SL. Variables related to sleep problems in children with autism. Res Autism Spectr Discord. 2009;3(4):931–941. [Google Scholar]
- 20. May T, Cornish K, Conduit R, Rajaratnam SMW, Rinehart NJ. Sleep in high‐functioning children with autism: longitudinal developmental change and associations with behavior problems. Behav Sleep Med. 2015;13(1):2–18. [DOI] [PubMed] [Google Scholar]
- 21. Schreck K. Sleep problems as possible predictors of intensified symptoms of autism. Res Dev Disabil. 2004;25(1):57–66. [DOI] [PubMed] [Google Scholar]
- 22. Limoges É, Mottron L, Bolduc C, Berthiaume C, Godbout R. Atypical sleep architecture and the autism phenotype. Brain. 2005;128(5):1049–1061. [DOI] [PubMed] [Google Scholar]
- 23. Buckley AW, Rodriguez AJ, Jennison K, Buckley J, Thurm A, Sato S, et al. Rapid eye movement sleep percentage in children with autism compared with children with developmental delay and typical development. Arch Pediatr Adolesc Med. 2010;164(11):1032–1037. http://archpedi.jamanetwork.com/article.aspx?doi=10.1001/archpediatrics.2010.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ming X, Sun YM, Nachajon RV, Brimacombe M, Walters AS. Prevalence of parasomnia in autistic children with sleep disorders. Clin Med Pediatr. 2009;3:CMPed.S1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thirumalai SS, Shubin RA, Robinson R. Rapid eye movement sleep behavior disorder in children with autism. J Child Neurol. 2002;17(3):173–178. [DOI] [PubMed] [Google Scholar]
- 26. Gooley JJ, Chamberlain K, Smith KA, Khalsa SB, Rajaratnam SM, Van Reen E, et al. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab. 2011;96(3):463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kulman G, Lissoni P, Rovelli F, Roselli MG, Brivio F, Sequeri P. Evidence of pineal endocrine hypofunction in autistic children. Neuro Endocrinol Lett. 2000;21(1):31–34. [PubMed] [Google Scholar]
- 28. Nir I, Meir D, Zilber N, Knobler H, Hadjez J, Lerner Y. Brief report: circadian melatonin, thyroid‐stimulating hormone, prolactin, and cortisol levels in serum of young adults with autism. J Autism Dev Disord. 1995;25(6):641–654. [DOI] [PubMed] [Google Scholar]
- 29. Aoki Y, Yoncheva YN, Chen B, Nath T, Sharp D, Lazar M, et al. Association of white matter structure with autism spectrum disorder and attention‐deficit/hyperactivity disorder. JAMA Psychiatry. 2017;74(11):1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rommelse NN, Geurts HM, Franke B, Buitelaar JK, Hartman CA. A review on cognitive and brain endophenotypes that may be common in autism spectrum disorder and attention‐deficit/hyperactivity disorder and facilitate the search for pleiotropic genes. Neurosci Biobehav Rev. 2011;35(6):1363–1396. [DOI] [PubMed] [Google Scholar]
- 31. Tamon H, Fujino J, Itahashi T, Frahm L, Parlatini V, Aoki YY, et al. Shared and specific neural correlates of attention deficit hyperactivity disorder and autism spectrum disorder: a meta‐analysis of 243 task‐based functional mri studies. Am J Psychiatry. 2024;181(6):541–552. [DOI] [PubMed] [Google Scholar]
- 32. Bioulac S, Taillard J, Philip P, Sagaspe P. Excessive daytime sleepinessmeasurements in children with attention deficit hyperactivity disorder. Front Psychiatry. 2020;11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shelton AR, Malow B. Neurodevelopmental disorders commonlypresenting with sleep disturbances. Neurotherapeutics. 2021;18(1):156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Food and Drug Administration . HIGHLIGHTS OF PRESCRIBINGINFORMATION CONCERTA® (methylphenidate HCl) Extended-ReleaseTablets CII [Internet]. Maryland: Food and Drug Administration; 2017. [cited 2024 Mar 25] Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021121s038lbl.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


