Abstract
Introduction:
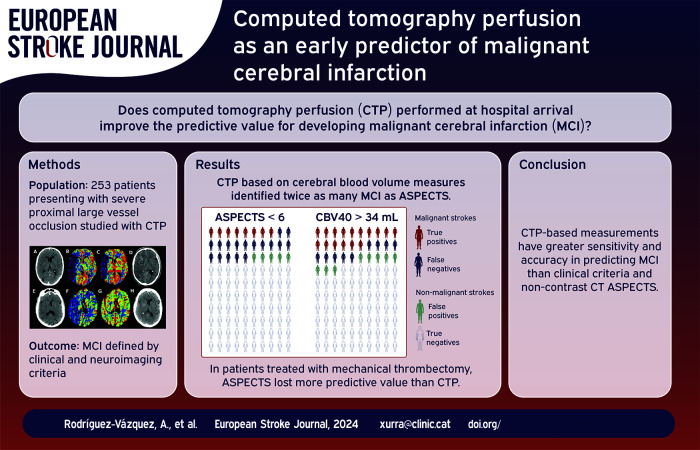
Malignant middle cerebral artery infarction (MCI) needs rapid intervention. This study aimed to enhance the prediction of MCI using computed tomography perfusion (CTP) with varied quantitative benchmarks.
Materials and Methods:
We retrospectively analyzed 253 patients from a single-center registry presenting with acute, severe, proximal large vessel occlusion studied with whole-brain CTP imaging at hospital arrival within the first 24 h of symptoms-onset. MCI was defined by clinical and imaging criteria, including decreased level of consciousness, anisocoria, death due to cerebral edema, or need for decompressive craniectomy, together with midline shift ⩾6 mm, or infarction of more than 50% of the MCA territory. The predictive accuracy of baseline ASPECTS and CTP quantifications for MCI was assessed by receiver operating characteristic (ROC) area under the curve (AUC) while F-score was calculated as an indicator of precision and sensitivity.
Results:
Sixty-three out of 253 patients (25%) fulfilled MCI criteria and had worse clinical and imaging results than the non-MCI group. The capacity to predict MCI was lower for baseline ASPECTS (AUC 0.83, F-score 0.52, Youden’s index 6), than with perfusion-based measures: relative cerebral blood volume threshold <40% (AUC 0.87, F-score 0.71, Youden’s index 34 mL) or relative cerebral blood flow threshold <35% (AUC 0.87, F-score 0.62, Youden’s index 67 mL). CTP based on rCBV measurements identified twice as many MCI as baseline CT ASPECTS.
Discussion and conclusion:
CTP-based quantifications may offer enhanced predictive capabilities for MCI compared to non-contrast baseline CT ASPECTS, potentially improving the monitoring of severe ischemic stroke patients at risk of life-threatening edema and its treatment.
Keywords: Computed tomography perfusion, malignant cerebral infarction, brain edema, acute stroke
Graphical abstract.
Introduction
Ischemic stroke stands as the second leading cause of death worldwide and a significant contributor to disability. 1 Approximately 10% of ischemic strokes follow a “malignant” course, primarily defined by extensive infarction within the middle cerebral artery (MCA) territory. 2 Such strokes rapidly progress to cerebral edema, leading to mass effects that may culminate in brain herniation and death. 3 Notably, younger patients lack cerebral atrophy, predisposing them to this severe outcome. 4
Current medical interventions have yet to demonstrate efficacy in altering the natural progression of malignant infarctions.5–9 To date, the only beneficial treatment is timely decompressive surgery, which has been shown to enhance survival, albeit often with residual moderate to severe disability.10–14 Predictive models for malignant transformation traditionally rely on magnetic resonance imaging (MRI), which may not be universally available. 15 Computed tomography perfusion (CTP), while more accessible, has often been less precise than MRI for assessing ischemic damage. 16 Recent research, however, suggests that specific gray and white matter perfusion thresholds may enhance CTP’s predictive accuracy. 17
With new clinical trials expanding the use of mechanical thrombectomy in patients with low ASPECTS scores,18–23 there is a growing need to identify individuals at high risk for malignant progression. This study evaluated the predictive value of acute CTP findings at hospital arrival for developing malignant cerebral infarction (MCI) in the following hours, compared with other potential clinical and radiological predictors of this severe complication.
Methods
Patient selection
Data for this retrospective study were systematically extracted from our local stroke database, which prospectively compiles each patient’s medical record. We assessed 804 patients considered for stroke reperfusion therapy from 2010 to 2017 and identified 253 patients at heightened risk for MCI, with the following inclusion criteria:
1. Proximal large vessel occlusion involving the carotid artery, M1 segment, or M2 segment (if the basal ganglia were affected) of the MCA.
2. Acute hemispheric syndrome with severe symptoms (National Institute of Health Stroke Scale (NIHSS) > 15 for dominant hemisphere or NIHSS > 13 for non-dominant hemisphere) within 24 h post-admission.
3. Admission multimodal CT protocol, including non-contrast CT (NCCT), CT angiography (CTA), and CTP.
4. Follow-up neuroimaging (CT or MRI) conducted within 72 h of admission.
5. Clinical evaluations conducted 24–72 h post-admission and at a 3-month follow-up.
Demographic, clinical, and blood test variables were collected for each patient, along with the time from symptom onset to neuroimaging, occlusion site, type of revascularization treatment, and the degree of final recanalization. The clinical severity at admission and in the first 24 h was addressed using the NIHSS, and the functional outcome at 3 months was addressed using the modified Rankin scale (mRS). As an observational cohort study, this work adheres to the STROBE guidelines. 24
Study outcomes – definition of MCI
The principal outcome, MCI, was defined through a combination of clinical and neuroimaging criteria2,4,25:
1. Clinical indicators of intracranial hypertension, such as a decreased level of consciousness (score ⩾ 1 in the corresponding item on NIHSS), anisocoria, death due to cerebral edema, or need for decompressive craniectomy.
2. Neuroimaging evidence indicating significant cerebral edema, exemplified by a midline shift ⩾6 mm or an infarct encompassing over half of the MCA territory.
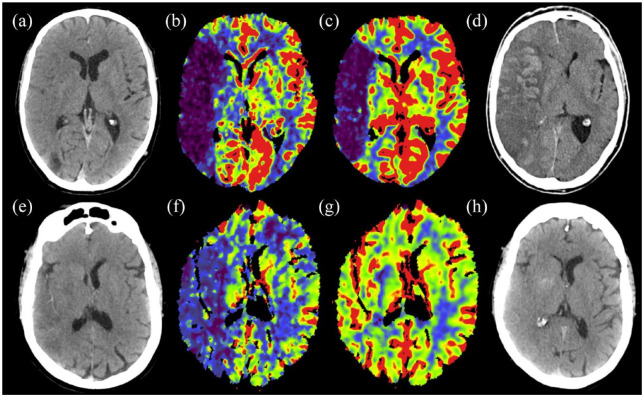
A diagnosis of malignant stroke required the presence of at least one clinical and one neuroimaging criterion. An example of MCI is shown in Figure 1.
Figure 1.
Non-contrast CT scan (a), rCBF (b), and rCBV (c) maps of a 63-year-old man with an acute stroke due to an occlusion of the M1 segment of the right middle cerebral artery. Complete recanalization was obtained with mechanical thrombectomy. CT performed 24 h later (d) showed malignant cerebral edema with 12 mm midline shift. Non-contrast CT scan (e), rCBF (f), and rCBV (g) maps of a 60-year-old female with an acute stroke due to right MCA M1 occlusion and complete recanalization after mechanical thrombectomy. CT performed 24 h later (h) showed mild edema without a midline shift. Note the difference in the perfusion maps, with a greater decrease in CBF and, especially, CBV in the first case, that developed malignant cerebral infarction.
Neuroimaging analysis
The imaging protocol included a baseline multimodal whole-brain CT scan (total acquisition time, 83 seconds), which included NCCT (140 kV, 127 mAs, FOV = 225 mm, matrix = 512 × 512, section thickness = 5 mm); CTA (120 kV, 663 mAs, FOV = 261 mm, matrix = 512 × 512, section thickness = 0.6 mm); and CTP (80 kV[peak], 250 mAs, 1.5-s rotation, FOV = 18 mm, matrix = 512 × 512, and forty-nine 2-mm-thickness slices). Patients were scanned using a Somatom Definition Flash 128-section dual-source multidetector scanner (Siemens), with a 98 mm z-coverage and 26 time points acquired each 1.5 s and 4 last time points each 5 s (total acquisition time, 59 s). Fifty milliliters of nonionic iodinated contrast were administered intravenously at 5 mL/s using a power injector, followed by a saline flush of 20 mL at an injection rate of 2 mL/s.
The ASPECTS was assessed on the baseline NCCT. CTP maps were calculated by the commercial software MIStar (Apollo Medical Imaging Technology) using a model-free singular-value decomposition algorithm with a delay and dispersion correction. The software automatically performs motion correction and selects an arterial input function from an unaffected artery (usually the anterior cerebral artery) and a venous output function from a large draining vein (the sagittal sinus). The software generates cerebral blood flow (CBF), cerebral blood volume (CBV), mean transient time (MTT), and delay time (DT) maps. Of note, the delay-corrected deconvolution method produces delay time maps rather than the more extensively used time-to-maximum maps. 26 A threshold of 3 s on the delay time maps was used to define the hypoperfusion, 27 and ischemic core was defined within the hypoperfused area with a series of relative CBF (rCBF) and CBV (rCBV) thresholds as a percentage of the mean perfusion values from the entire unaffected, contralateral hemisphere. 16 Estimates of the ischemic area were made using all rCBF and rCBV thresholds from 0% to 100%.
The follow-up imaging was performed on the same NCCT or on MRI (1.5T Magneton Aera unit, Siemens). Midline shift was measured as the perpendicular distance between the septum pellucidum and a line drawn between the anterior and posterior attachments of the falx to the inner table of the skull.
Two trained observers reviewed neuroimaging and clinical information for MCI evaluation, and a third acted as a referee in case of disagreement. A single observer assessed CTP calculations semiautomatically.
Statistical analysis
The statistical procedures were executed using IBM SPSS Statistics, version 26.0 (IBM Corp., Armonk, NY), and Python 3.8.5 with the Numpy, Pandas, Scikit-learn, Matplotlib, and Seaborn libraries.
Categorical variables were assessed using the Chi-square (χ2) test when appropriate, with Fisher’s exact test as an alternative for small sample sizes. Continuous variables following a normal distribution were presented using means and standard deviations (SD), whereas those not normally distributed, along with ordinal variables, were described using medians and interquartile ranges (IQR). Group comparisons for means and medians were conducted via the Student’s t-test, ANOVA, or Mann-Whitney U test, contingent upon the data’s distribution. The relationship between perfusion patterns and clinical outcomes was evaluated using univariate logistic regression. The train test split function was employed for splitting datasets into training and test sets, ensuring a robust validation of the models’ performance. To assess the predictive value, accuracy, and classification performance of various clinical and radiological variables for malignant stroke, we employed logistic regression models to evaluate individual predictors, including NIHSS, ASPECTS, and all perfusion parameters. Each variable’s performance was quantified by calculating the Area Under the Receiver Operating Characteristic (AUC) curve, which reflects the model’s ability to distinguish between malignant and non-malignant stroke cases with a higher AUC indicating better model performance. Accuracy was reported as the proportion of correct predictions over the total cases. Sensitivity, or the true positive rate, was prioritized to emphasize the correct identification of malignant stroke due to its clinical significance in prognosis and treatment planning. In addition, precision was calculated to assess the proportion of actual malignant stroke cases among those predicted as such, thereby reflecting the model’s ability to produce a low rate of false positives. The F1-score, a harmonic mean of precision and sensitivity, was also determined to evaluate the model’s balance between identifying true malignant cases (sensitivity) and avoiding false positive diagnoses (precision). These metrics provided a comprehensive view of the model’s performance, especially considering the imbalanced nature of the dataset, where malignant strokes were less prevalent than non-malignant strokes. The election of optimal single cutoff values was based on Youden’s J statistic, which maximizes the true positive rate (sensitivity) and minimizes the false positive rate. Significance was set at p < 0.05 for all tests, and hypotheses were bilateral.
Data Availability
Datasets produced and analyzed during this study can be obtained from the corresponding author upon a reasonable request.
Results
Out of 253 patients who satisfied the inclusion criteria (Figure 2), 63 individuals (25%) were classified as having MCI. The demographic and pre-stroke clinical characteristics – such as sex, age, and clinical history – were comparable between the MCI and non-MCI groups, as was the interval from symptom onset to initial imaging.
Figure 2.
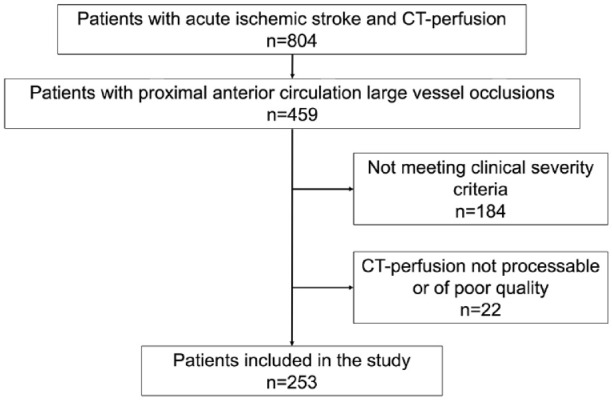
Flow chart of the patients included in the study.
As shown in Table 1, patients with MCI exhibited a higher baseline glycemia, lower baseline ASPECTS scores, more carotid and tandem occlusions, and higher NIHSS scores at admission and after 24 h. Reperfusion treatments, such as mechanical thrombectomy with or without intravenous alteplase, were more frequently administered in the non-MCI group, which also showed superior angiographic results. Hemorrhagic transformations occurred more often in the MCI group. At 3 months, the median mRS scores and mortality rates were higher in the MCI group.
Table 1.
Clinical characteristics of the included patients according to the development of malignant MCA infarction.
| Total n = 253 | Non-malignant n = 190 (75%) | Malignant n = 63 (25%) | p-Value | |
|---|---|---|---|---|
| Female, n (%) | 129 (51) | 98 (51) | 32 (50) | 0.74 |
| Age (years), median (IQR) | 75 (62–81) | 75 (62–81) | 75 (64–81) | 0.91 |
| Premorbid mRS, median (IQR) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.22 |
| Hypertension, n (%) | 165 (65) | 122 (64) | 43 (68) | 0.55 |
| Dyslipidemia, n (%) | 114 (45) | 84 (44) | 30 (47) | 0.63 |
| Diabetes mellitus, n (%) | 53 (21) | 42 (22) | 11 (17) | 0.43 |
| Smoker, n (%) | 41 (16) | 31 (16) | 10 (15) | 0.93 |
| Ischemic cardiopathy, n (%) | 44 (17) | 31 (16) | 13 (20) | 0.43 |
| Atrial fibrillation, n (%) | 74 (29) | 56 (29) | 18 (28) | 0.91 |
| Previous stroke, n (%) | 23 (9) | 17 (8) | 6 (9) | 0.89 |
| Occlusion site | <0.001 | |||
| M1, n (%) | 132 (52) | 105 (55) | 27 (42) | |
| M2, n (%) | 53 (21) | 52 (27) | 1 (1) | |
| ICA, n (%) | 30 (11) | 14 (7) | 16 (25) | |
| Tandem, n (%) | 38 (15) | 19 (10) | 19 (30) | |
| ASPECTS, median (IQR) | 9 (7–10) | 9 (1–10) | 7 (6–8) | 0.001 |
| NIHSS at admission, median (IQR) | 17 (12–21) | 16 (10–20) | 19 (17–22) | 0.001 |
| NIHSS at 24 h, median (IQR) | 11 (5–11) | 7 (3–15) | 21 (18–25) | <0.001 |
| Treatment | <0.001 | |||
| None, n (%) | 24 (9) | 16 (8) | 8 (12) | |
| rtPA, n (%) | 52 (20) | 29 (15) | 23 (36) | |
| MT, n (%) | 73 (28) | 53 (27) | 20 (31) | |
| MT + rtPA, n (%) | 104 (41) | 92 (48) | 12 (19) | |
| TICI | N = 176 | N = 145 | N=31 | 0.001 |
| 0, n (%) | 20 (11) | 12 (8) | 8 (25) | |
| 1, n (%) | 4 (2) | 4 (2) | 0 (0) | |
| 2a, n (%) | 19 (10) | 13 (6) | 6 (19) | |
| 2b, n (%) | 56 (31) | 45 (23) | 11 (35) | |
| 2c–3, n (%) | 77 (43) | 71 (37) | 6 (19) | |
| Time-to-CTP in hours, median (IQR) | 2.98 (1.75–4.78) | 2.94 (1.61–4.76) | 3.18 (1.88–5.46) | 0.40 |
| Basal glycemia (mg/dL), mean (SD) | 122 (106–144) | 118 (101–142) | 127 (114–148) | 0.008 |
| Systolic blood pressure (mmHg), median (IQR) | 127 (114–148) | 140 (130–156) | 142 (125–165) | 0.40 |
| Hemorrhagic transformation | 0.001 | |||
| None, n (%) | 170 (67) | 135 (71) | 35 (50) | |
| HI1, n (%) | 14 (5) | 10 (5) | 4 (6) | |
| HI2, n (%) | 19 (7) | 15 (7) | 4 (6) | |
| PH1, n (%) | 17 (6) | 13 (6) | 4 (6) | |
| PH2, n (%) | 11 (4) | 2 (1) | 9 (14) | |
| SAH, n (%) | 20 (7) | 14 (7) | 6 (9) | |
| Symptomatic hemorrhagic transformation, n% | 10 (3) | 2 (1) | 8 (4) | <0.001 |
| TOAST | 0.80 | |||
| LAA, n (%) | 41 (16) | 28 (14) | 13 (20) | |
| Cardioembolism, n (%) | 125 (49) | 97 (51) | 28 (44) | |
| Undetermined, n (%) | 70 (27) | 52 (27) | 18 (28) | |
| Other, n (%) | 15 (5) | 11 (5) | 4 (6) | |
| mRS at 90 days, median (IQR) | 3 (1–4) | 2 (1–4) | 6 (4–6) | <0.001 |
IQR: interquartile range; mRS: modified Rankin scale; M1: M1 segment of the middle cerebral artery; ICA: internal carotid artery; M2: M2 segment of the middle cerebral artery; ASPECTS: Alberta Stroke Program Early CT Score; NIHSS: National Institute of Health Stroke Scale; rtPA: recombinant tissue plasminogen activator; MT: mechanical thrombectomy; TICI: thrombolysis in cerebral infarction scale; CTP: computed tomography perfusion; mg/dL: milligrams per deciliter; mmHg: millimeters of mercury; SD: standard deviation; HI: hemorrhagic infarction; PH: parenchymal hematoma; SAH: subarachnoidal hemorrhage; TOAST: Trial of Org 10172 in Acute Stroke Treatment; LAA: large-artery atherosclerosis.
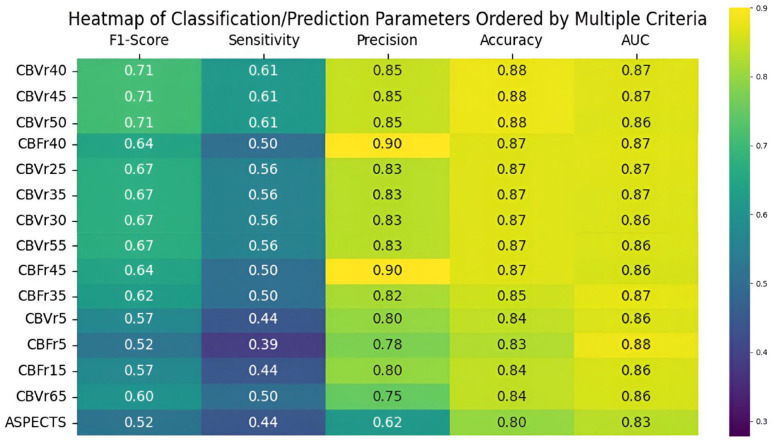
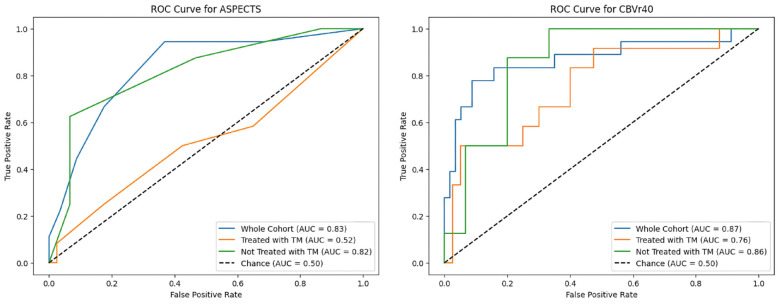
Regarding the predictive capacity for MCI, baseline ASPECTS had a ROC AUC of 0.83 and F1-score of 0.52 for a cut-off lower than 6, which is also the limit traditionally used when considering treatment with mechanical thrombectomy. However, several CTP parameters outperformed it (Figure 3). Values based on cerebral blood volume performed best, with AUC of 0.87 and F1-scores of 0.71 for rCBV thresholds lower than 40%, with the best volume cut-off for CBV being 34 mL. Regarding cerebral blood flow, the most robust predictor was found to be a rCBF threshold of less than 35% for a 67 mL cut-off.
Figure 3.
Heatmap summarizing the predictive performance of ASPECTS and several perfusion-based metrics ordered by decreasing F1-scores.
rCBF: relative cerebral blood flow; rCBV: relative cerebral blood volume.
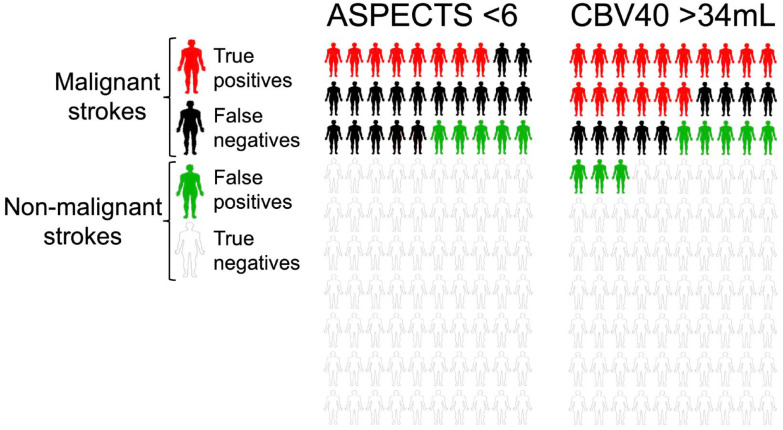
In our cohort of 253 patients, ASPECTS < 6 identified 19 true positives for MCI compared to 38 matched by the 34 mL rCBV 40% threshold. False negatives were lower using CTP compared to ASPECTS (24 vs 43), with a slight increase in false positives (20 vs 12). A diagram of these statistical variables is shown in Figure 4. There were no significant differences between observers (Kappa coefficient for the definition of malignant infarction: 0.93, intraclass correlation coefficient for the quantitative assessment of midline shift: 0.99).
Figure 4.
Diagram of the distribution of true positives, false negatives, false positives, and true negatives using ASPECTS cut-off values <6 and CTP rCBV 40% >34 mL. Considering the 25% prevalence of MCI in our study cohort, ASPECTS identified 8 true positives, with 17 false negatives and 5 false positives. The rCBF cut-off identified 16 true positives, with 9 false negatives, and 8 false positives.
Given the asymmetry between the proportion of patients in each group (malignant and non-malignant) undergoing mechanical thrombectomy, a specific analysis was performed using ROC evaluating the ability of ASPECTS and the CTP rCBV 40% to differentiate between true and false positives according to the use of mechanical thrombectomy (Figure 5). ASPECTS lost most of its predictive value in patients treated with thrombectomy (AUC of 0.82 in untreated patients, 0.52 in treated patients), while the decrease in predictive value was milder for rCBV 40% (AUC of 0.86 and 0.76 in untreated and treated patients, respectively).
Figure 5.
ROC curve of ASPECTS and CT-perfusion rCBV 40% in the whole cohort, patients who underwent mechanical thrombectomy and patients not treated with mechanical thrombectomy.
Discussion
In our cohort of patients with moderate to severe symptoms at first evaluation or during the first 24 h after admission, CTP-based measurements outperformed those based only in NCCT ASPECTS for predicting malignant infarction. The pathogenesis of MCI is multifaceted. Severe presenting symptoms are well-recognized clinical harbingers of MCI.2,4 While MRI is traditionally favored for its precision in quantifying infarct volume – especially volumes exceeding 145 mL on DWI – its predictive reliability hinges on the ADC thresholds applied.15,27 MRI’s sensitivity and specificity are heightened in evaluations conducted 24 h post-symptom onset rather than in hyperacute assessments. 28 Although CT is less sensitive in the early phase, its greater accessibility and the augmentation of its predictive power through multimodal CT perfusion imaging are noteworthy. 29 Our investigation focused on multimodal CT scans executed upon admission, with a sub-6-h window from symptom onset, aiming to swiftly pinpoint patients at elevated risk for MCI to expedite intervention. We purposely selected a cohort of patients at high risk of developing MCI, so the rate of malignant infarctions was 25%, higher than the 10% classically described in the general population. 2
In patients with extensive symptoms, the optimal CTP thresholds for predicting malignant infarction were higher than the commonly used <30% threshold tied to DWI-MRI ischemic core definitions. Although DWI is traditionally used as the gold standard to define the extent of the ischemic lesion as an element related to the functional outcome,30,31 the relevance of cerebral edema in the development of MCI – and potentially influencing these threshold discrepancies – cannot be understated.32,33
Compared to rCBF thresholds, rCBV showed slightly greater ability to predict MCI in our cohort. This may be because the volume drop is indicative of greater cerebral hypoperfusion, which could lead to greater development of cerebral edema and more ischemic tissue. Regardless of whether the measurement was based on in flow or volume, CTP-based models present greater sensitivity, precision, and accuracy than NCCT ASPECTS. Thus, considering the extreme clinical severity of MCI, it is especially important to use a tool that can identify the greatest number of patients at risk. In our cohort, CTP performed before any type of treatment identified twice as many patients who developed MCI compared to ASPECTS with half the number of false negatives and anincrease in false positives.
In routine clinical practice, the greater predictive capacity of models based on cerebral perfusion compared to NCCT ASPECTS could be useful for optimizing the management circuits of patients with acute ischemic strokes. For example, using the information provided by CTP to identify patients at high risk of MCI could serve to carry out more strict surveillance protocols or early control neuroimaging that would not be performed under normal conditions.
The strength of this work lies in its simple design that is applicable to routine clinical practice, based on a proven and widely accepted imaging methodology. In this way, it is an easy element to apply regardless of the available resources and the clinical severity of the patient.
On the other hand, the study’s retrospective design, despite the prospective nature of data collection, may introduce limitations. The fact that it is a single-center study is also a limitation, although the accessibility of the technique favors potential reproducibility. Our data predate the routine consideration of mechanical thrombectomy for extensive strokes, potentially skewing outcomes for patients presenting with ASPECTS under 6 due to the infrequency of intervention at that time. Considering the use of the ASPECTS as a tool to choose patients eligible for treatment, this bias, might have overestimated the predictive capacity of the ASPECTS as patients with large infarcts and large vessel occlusions that do not recanalize are at higher risk of MCI. With the increasing inclination toward reperfusion therapies for extensive ischemic lesions, our findings could prove instrumental in identifying patients at increased risk for malignant cerebral edema if confirmed in patients with substantial infarct cores subjected to endovascular therapy.
Conclusions
CTP-based measurements showed heightened sensitivity and accuracy in predicting MCI compared to clinical criteria and NCCT ASPECTS. This approach could offer utility in identifying high-risk patients, potentially enabling more comprehensive monitoring and expediting the management of life-threatening complications in severe ischemic stroke patients. A prospective study with data from more than one center would be useful to confirm these findings.
Acknowledgments
We sincerely thank Martha Vargas for supporting data collection.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Spanish Ministry of Health cofinanced by the European Regional Development Fund (Instituto de Salud Carlos III, Red Temática de Investigación Cooperativa Invictus+; RD16/0019/0014) and grant PI21/00966 to Dr. Urra and Dr. Chamorro. Dr. Rodríguez-Vázquez receives funding from Instituto de Salud Carlos III, with a Grant for Health Research (CM22/00068). Xabier Urra is sponsored by the Instituto de Salud Carlos III, reference number INT22/00043, and by the European Social Fund «The ESF – Investing in your future». Dr. Rudilosso receives funding from Instituto de Salud Carlos III, with a grant for health research (JR21/00011). This work was partially developed at the building Centro Esther Koplowitz, Barcelona, CERCA Program/Generalitat de Catalunya.
Ethical approval: The local Clinical Research Ethics Committee from Hospital Clínic Barcelona approved the study protocol (registration number HCB/2020/0233) under the requirements of national legislation.
Informed consent: Informed consent was not sought for this study due to its retrospective nature.
Disclosures: This work has been accepted for presentation at the 10th European Stroke Organisation Conference (May 2024, Basel, Switzerland).
Guarantor: ARV, XU
Contributorship: ARV, XU: study concept and design; major role in the acquisition of data; analysis and interpretation of data; drafting of the manuscript. CL: major role in the acquisition of data; analysis and interpretation of data. ADM, LR, LLla: major role in the acquisition of data. All authors interpreted the results, reviewed the manuscript and substantially contributed to the final manuscript.
ORCID iDs: Alejandro Rodríguez-Vázquez  https://orcid.org/0000-0002-3448-9778
https://orcid.org/0000-0002-3448-9778
Guillem Dolz  https://orcid.org/0009-0002-7712-0193
https://orcid.org/0009-0002-7712-0193
Salvatore Rudilosso  https://orcid.org/0000-0002-8775-6884
https://orcid.org/0000-0002-8775-6884
References
- 1. Campbell BCV, De Silva DA, Macleod MR. Ischaemic stroke. Nat Rev Dis Primers 2019; 5: 71. [DOI] [PubMed] [Google Scholar]
- 2. Hacke W, Schwab S, Horn M, et al. Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol 1996; 53: 309–315. [DOI] [PubMed] [Google Scholar]
- 3. White OB, Norris JW, Hachinski VC, et al. Death in early stroke, causes and mechanisms. Stroke 1979; 10: 743. [DOI] [PubMed] [Google Scholar]
- 4. Huttner HB, Schwab S. Malignant middle cerebral artery infarction: clinical characteristics, treatment strategies, and future perspectives. Lancet Neurol 2009; 8: 949–958. [DOI] [PubMed] [Google Scholar]
- 5. Simard JM, Sahuquillo J, Sheth KN, et al. Managing malignant cerebral infarction. Curr Treat Options Neurol 2011; 13: 217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhardwaj A, Harukuni I, Murphy SJ, et al. Hypertonic saline worsens infarct volume after transient focal ischemia in rats. Stroke 2000; 31: 1694–1701. [DOI] [PubMed] [Google Scholar]
- 7. Cruz J, Minoja G, Okuchi K, et al. Successful use of the new high-dose mannitol treatment in patients with Glasgow coma scale scores of 3 and bilateral abnormal pupillary widening: a randomized trial. J Neurosurg 2004; 100: 376–383. [DOI] [PubMed] [Google Scholar]
- 8. Koenig MA 3rd, Bryan M, Lewin JL. Re: reversal of transtentorial herniation with hypertonic saline. Neurology 2009; 72: 200–209. [DOI] [PubMed] [Google Scholar]
- 9. Olsen TS, Weber UJ, Kammersgaard LP. Therapeutic hypothermia for acute stroke. Lancet Neurol 2003; 2: 410–416. [DOI] [PubMed] [Google Scholar]
- 10. Vahedi K, Hofmeijer J, Juettler E, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol 2007; 6: 215–222. [DOI] [PubMed] [Google Scholar]
- 11. Hofmeijer J, Kappelle LJ, Algra A, et al. Surgical decompression for space-occupying cerebral infarction (the hemicraniectomy after middle cerebral artery infarction with life-threatening edema trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol 2009; 8: 326–333. [DOI] [PubMed] [Google Scholar]
- 12. Vahedi K, Vicaut E, Mateo J, et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL trial). Stroke 2007; 38: 2506–2517. [DOI] [PubMed] [Google Scholar]
- 13. Jüttler E, Schwab S, Schmiedek P, et al. Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery (DESTINY): a randomized, controlled trial. Stroke 2007; 38: 2518–2525. [DOI] [PubMed] [Google Scholar]
- 14. Jüttler E, Bösel J, Amiri H, et al. DESTINY II: DEcompressive surgery for the treatment of malignant INfarction of the middle cerebral arterY II. Int J Stroke 2011; 6: 79–86. [DOI] [PubMed] [Google Scholar]
- 15. Thomalla G, Hartmann F, Juettler E, et al. Prediction of malignant middle cerebral artery infarction by magnetic resonance imaging within 6 hours of symptom onset: a prospective multicenter observational study. Ann Neurol 2010; 68: 435–445. [DOI] [PubMed] [Google Scholar]
- 16. Laredo C, Renú A, Tudela R, et al. The accuracy of ischemic core perfusion thresholds varies according to time to recanalization in stroke patients treated with mechanical thrombectomy: a comprehensive whole-brain computed tomography perfusion study. J Cereb Blood Flow Metab 2020; 40: 966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodríguez-Vázquez A, Laredo C, Renú A, et al. Optimizing the definition of ischemic core in CT perfusion: influence of infarct growth and tissue-specific thresholds. AJNR Am J Neuroradiol 2022; 43: 1265–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshimura S, Sakai N, Yamagami H, et al. Endovascular therapy for acute stroke with a large ischemic region. New Engl J Med 2022; 386: 1303–1313. [DOI] [PubMed] [Google Scholar]
- 19. Huo X, Ma G, Tong X, et al. Trial of endovascular therapy for acute ischemic stroke with large infarct. New Engl J Med 2023; 388: 1272–1283. [DOI] [PubMed] [Google Scholar]
- 20. Sarraj A, Hassan AE, Abraham MG. Endovascular thrombectomy for acute large ischemic strokes. New Engl J Med 2023; 389: 88–90. [DOI] [PubMed] [Google Scholar]
- 21. Bendszus M, Fiehler J, Subtil F, et al. Endovascular thrombectomy for acute ischaemic stroke with established large infarct: multicentre, open-label, randomised trial. Lancet 2023; 402: 1753–1763. [DOI] [PubMed] [Google Scholar]
- 22. Zaidat OO, Yoo AJ. On behalf of TESLA investigators TESLA trial: Primary results. In: Proceedings of the European stroke organisation conference 2023, Munich, Germany, 26 May 2023. [Google Scholar]
- 23. Costalat V, Lapergue B, Albucher JF, et al. Evaluation of acute mechanical revascularization in large stroke (ASPECTS 5) and large vessel occlusion within 7 h of last-seen-well: the LASTE multicenter, randomized, clinical trial protocol. Int J Stroke 2024; 19: 114–119. [DOI] [PubMed] [Google Scholar]
- 24. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 25. Treadwell SD, Thanvi B. Malignant middle cerebral artery (MCA) infarction: pathophysiology, diagnosis and management. Postgrad Med J 2010; 86: 235–242. [DOI] [PubMed] [Google Scholar]
- 26. Bivard A, Levi C, Spratt N, et al. Perfusion CT in acute stroke: a comprehensive analysis of infarct and penumbra. Radiology 2013; 267: 543–550. [DOI] [PubMed] [Google Scholar]
- 27. Oppenheim C, Samson Y, Manaï R, et al. Prediction of malignant middle cerebral artery infarction by diffusion-weighted imaging. Stroke 2000; 31: 2175–2181. [DOI] [PubMed] [Google Scholar]
- 28. Kruetzelmann A, Hartmann F, Beck C, et al. Combining magnetic resonance imaging within six-hours of symptom onset with clinical follow-up at 24 h improves prediction of ‘malignant’ middle cerebral artery infarction. Int J Stroke 2014; 9: 210–214. [DOI] [PubMed] [Google Scholar]
- 29. Ryoo JW, Na DG, Kim SS, et al. Malignant middle cerebral artery infarction in hyperacute ischemic stroke: evaluation with multiphasic perfusion computed tomography maps. J Comput Assist Tomogr 2004; 28: 55–62. [DOI] [PubMed] [Google Scholar]
- 30. Lin L, Bivard A, Krishnamurthy V, et al. Whole-brain CT perfusion to quantify acute ischemic penumbra and Core. Radiology 2016; 279: 876–887. [DOI] [PubMed] [Google Scholar]
- 31. Campbell BC, Purushotham A, Christensen S, et al. The infarct core is well represented by the acute diffusion lesion: sustained reversal is infrequent. J Cereb Blood Flow Metab 2012; 32: 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Broocks G, Kemmling A, Kniep H, et al. Edema reduction versus penumbra salvage: investigating treatment effects of mechanical thrombectomy in ischemic stroke. Ann Neurol 2023; 95: 137–145. DOI: 10.1002/ana.26802. [DOI] [PubMed] [Google Scholar]
- 33. Huang X, Chen C, Wang H, et al. The ACORNS grading scale: a novel tool for the prediction of malignant brain edema after endovascular thrombectomy. J Neurointerv Surg 2023; 15: e190–e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets produced and analyzed during this study can be obtained from the corresponding author upon a reasonable request.