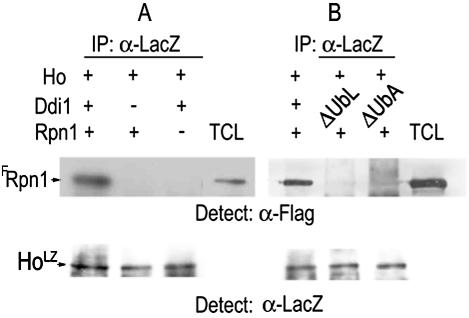
FIG. 3.
Ho forms a complex with FRpn1, and this requires both domains of Ddi1. (A) Protein A-HoLZ beads from IPs in which HoLZ was produced in the presence or the absence of Ddi1 were washed and then incubated with equal aliquots of FRpn1 lysate. HoLZ coimmunoprecipitates with FRpn1 only in the presence of Ddi1. (B) The above protein A-HoLZ beads were produced in extracts of cells producing Ddi1, LexA-Ddi1ΔUbL, or LexA-Ddi1ΔUbA as in Fig. 2D. They were incubated with equal aliquots of the FRpn1 lysate. The coimmunoprecipitated pellets were gel separated and blotted and anti-FLAG (α-FLAG) was used to detect FRpn1. No complex is formed between Ho and Rpn1 in the absence of either the UbL or the UbA domain of Ddi1. Total cell lysate (TCL) shows the FRpn1 band. Lower panel shows a control aliquot of HoLZ from each cell type by anti-LacZ IP-Western blot.