Abstract
Background
Advancements in flow diversion technology have revolutionized the treatment of intracranial aneurysms. The pipeline embolization device (PED) and the flow redirection endoluminal device (FRED) have emerged as prominent tools in this field. This study aims to compare the safety and efficacy profiles of PED and FRED in the treatment of intracranial aneurysms.
Methods
Following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines, a comprehensive literature search was conducted across PubMed, Web of Science, and Scopus databases. Studies comparing PED and FRED were included and data extraction focused on study characteristics, patient demographics, and clinical and radiological outcomes. Primary outcomes were favorable outcomes, described as modified Rankin scale (mRS) 0–2 score, and complete/near-complete occlusion, while secondary outcomes included retreatment rate and thromboembolic and hemorrhagic complications.
Results
Five studies, comprising 1238 patients, were included. No significant differences were found between PED and FRED in terms of complete occlusion at 6 months and 1 year, complete/near-complete occlusion at the last follow up, retreatment rates, and thromboembolic, in-stent thrombosis and hemorrhagic complications. However, FRED was significantly associated with higher favorable outcomes compared to PED (odds ratio: 0.37; confidence interval: 0.17 to 0.81; p = 0.01).
Conclusion
This study showed that both PED and FRED had comparable rates of complete occlusion, retreatment and complications, and FRED also demonstrated a higher likelihood of achieving favorable outcomes. The study underscores the need for further research with larger cohorts and longer follow up to consolidate these findings.
Keywords: Aneurysms, intracranial, flow redirection endoluminal device, pipeline embolization device, meta-analysis
Introduction
The treatment of intracranial aneurysms has evolved significantly over the past decade, particularly with the increasing adoption of flow diversion technology. 1 This shift has been substantiated by multiple clinical trials demonstrating the safety and efficacy of this approach.2–9 Unlike traditional coil packing methods which primarily target the aneurysm sac, flow diversion involves the endoluminal reconstruction of the parent vessel using a flow-diverting stent. 10 This technique has been pivotal in treating aneurysms that are challenging to address with conventional methods.10–12
In the United States, many flow-diverting devices have gained prominence including the pipeline embolization device (PED; Medtronic Inc.), which received FDA approval in 2011,2,8 and the flow redirection endoluminal device (FRED; MicroVention), approved in 2019.13–16 While many studies have reported experiences with PED, data on other devices like FRED are fewer. 17
Given the critical role these devices play in the management of intracranial aneurysms, it is essential to understand their comparative effectiveness and safety. Therefore, this study aims to conduct a systematic review and meta-analysis to evaluate the outcomes and safety profiles of PED and FRED devices.
Methods
Literature search
This study was prepared in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines. 18 A systematic search was performed on December 15, 2023, using the PubMed (National Library of Medicine), Web of science, and Scopus databases from inception to present. According to each database, different combinations of various keywords and/or Medical Heading Subjects are used, including intracranial, aneurysm, flow diverter, flow diversion, Pipeline, FRED. Only publications on human subjects and those published or professionally translated to English language were included. A full detailed search strategy for each database is shown in the Supplemental Table 1.
Study selection process
After completion of the search in three databases, the results were screened against title and abstract by two reviewers according to prespecified inclusion and exclusion criteria. Points of disagreement were resolved by consultation with a third author until consensus among the three authors was reached. Full texts were then screened to determine suitability for final inclusion. References of all included studies were searched to identify any additional studies that may have been missed during initial screening for inclusion. Inclusion criteria were: (1) comparative studies of PED and FRED and (2) reported outcomes of interest (occlusion rate and retreatment).
Exclusion criteria included duplicated studies, case reports, review articles, editorial letters, and conference abstracts.
Data extraction
The variables extracted from each study included title of article, year of publication, first author name, study design, sample size, patients’ demographics, comorbidities (hypertension (HTN) and diabetes mellitus (DM)), smoking, presence of multiple aneurysms, ruptured aneurysms, prior rupture, prior treatment, aneurysm location, aneurysm shape, aneurysm dimensions, number of flow diverters, flow diverters dimensions, fluoroscopy time, the use of adjunctive device, the use of antiplatelets, complete/near-complete occlusion, favorable outcomes, retreatment rate, thromboembolic, in-stent thrombosis and hemorrhagic complications, and follow-up period.
The primary outcomes were favorable outcomes and complete/near complete occlusion defined according to each included study. Secondary outcomes included retreatment rate and thromboembolic, in-stent thrombosis and hemorrhagic complications.
Risk of bias assessment
The Cochrane ROBINS-I tool was used to assess the risk of bias. 19 Each study was evaluated based on seven areas: confounding, selection of participants, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of reported result. The risk of bias was classed as low risk, moderate risk, serious risk, and critical risk.
Statistical analysis
Our meta-analysis was performed using R software (version 4.3.1, R Foundation for Statistical Computing, Vienna, Austria). In order to account for any variations in research design and patient demographic, a random-effects model was performed, taking into consideration the intrinsic methodological heterogeneity identified across the included studies. We calculated the mean difference (MD) for continuous data and the odds ratio (OR) for binary data. The MD was estimated using the inverse variance method, and the OR was estimated using the Mantel-Haenszel method. 20 For all statistical tests within the results synthesis, a p-value of <0.05 was used to indicate statistical significance. The presence of heterogeneity was assessed using I2 statistics and Q statistics, with heterogeneity considered significant when I2 exceeded 50% or the p-value was <0.05. In consideration of the limited number of studies, the evaluation of publication bias via Egger's regression test and assessment of the impact of sample size through meta-regression were not performed.
Results
Study identification
The primary study search resulted in 8549 articles, of which 4567 were duplicates. Of the 3982 screened articles, 20 articles were assessed in title, abstract, and full-text. After excluding nonrelevant studies, and studies that didn’t compare between PED and FRED, only five studies remain which were included in the final analysis21–25 (Figure 1). In terms of evaluating overall risk of bias in each study, all included studies had low risk of bias except for one (moderate risk) (Supplemental Figure 1A and B).
Figure 1.
PRISMA diagram.
Study characteristics
Tables 1 and 2 summarize the characteristics of the included studies. Five studies included 1238 patients, 873 of them (70.5%) were females. A meta-analysis was performed on the baseline characteristics. A significant difference was found between FRED and PED regarding the presence of multiple intracranial aneurysms (OR: 1.7; confidence interval (CI): 1.21 to 2.4, p < 0.01), the neck size of the aneurysm (MD: −0.66; CI: −1.27 to −0.05, p = 0.03), diameter of flow diversion (MD: −0.25; CI: −0.48 to −0.03, p = 0.03), length of flow diversion (MD: −5.35; CI: −7.39 to −3.31, p < 0.01), and the use of adjunctive coiling (OR: 0.19; CI: 0.04 to 0.83, p = 0.03) (Supplemental Figures 2, 3, 4, 5, and 6).
Table 1.
Baseline characteristics of studies included.
| Variables/studies/study designs/study period/countries | Field et al. (2023) | Griessenauer et al. (2020) | El Naamani et al. (2023) | Pichardo et al. (2020) | Griessenauer et al. (2018) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Retrospective cohort | Retrospective cohort | Retrospective cohort | Retrospective cohort | Retrospective cohort | ||||||
| 2018–2022 | 2012–2019 | 2018–2021 | 2016–2019 | 2013–2017 | ||||||
| USA | USA | USA | Mexico | USA | ||||||
| Type of flow diverter | PED | FRED | PED | FRED | PED | FRED | PED | FRED | PED | FRED |
| Number of flow diverter | 86 | 33 | 291 | 84 | 115 | 35 | 47 | 44 | 221 | 282 |
| Age, years (mean, SD) | 59.3 | 59.2 | 56.3 (13.4) | 55.7 (12.8) | 55.7 (12.8) | 60.9 (14.3) | 61.0 (11.0) | 61.0 (10.6) | 58.7 (11.9) | 54.7 (14.2) |
| Females | 73 | 22 | 147 | 42 | 98 | 30 | 26 | 24 | 191 | 220 |
| Hypertension | 41 | 9 | NA | NA | 52 | 14 | NA | NA | NA | NA |
| Smoking | 27 | 12 | 81 | 54 | 20 | 12 | NA | NA | NA | NA |
| Multiple aneurysms | NA | NA | 49 | 7 | NA | NA | NA | NA | 90 | 79 |
| Ruptured aneurysms | 16 | 0 | NA | NA | 10 | 3 | NA | NA | NA | NA |
| Prior rupture | 12 | 7 | 66 | 25 | 16 | 8 | NA | NA | NA | NA |
| Prior treatment | 20 | 7 | 27 | 7 | 24 | 14 | NA | NA | 31 | 15 |
| Anterior aneurysm location | 74 | 27 | 0 | 0 | 87 | 27 | 41 | 43 | 221 | 273 |
| Aneurysm shape, number | Saccular: 81 | Saccular: 31 | Saccular: 96, fusiform: 118, dissecting/blister: 77 | Saccular: 34, fusiform: 21, dissecting/blister: 29 | Saccular: 103 | Saccular: 27 | NA | NA | Saccular: 215, fusiform: 6 | Saccular: 257, fusiform: 16 |
| Neck size of aneurysm, mm | 4.3 (2) | 4.8 (2.3) | NA | NA | 3.7 (1.9) | 4.5 (2.3) | NA | NA | NA | NA |
| Width size of aneurysm, mm | 7.2 (4.7) | 7.5 (4.6) | NA | NA | 5.3 (4.3) | 7.1 (6.9) | NA | NA | NA | NA |
| Height size of aneurysm, mm | 8.2 (5) | 7.3 (5) | NA | NA | 5.1 (4.2) | 6.1 (5.3) | NA | NA | NA | NA |
| Diameter size of aneurysm, mm | 3.7 (0.7) | 4.0 (0.8) | 10.0 (6.7) | 6.7 (5.3) | NA | NA | 10.0 (4.8) | 11.0 (5.4) | 8.0 (5.2) | 6.7 (4.4) |
| Number of FD (mean, SD) | 1.15 (0.42) | 1.09 (0.38) | 1.15 (0.36) | 1.07 (0.37) | NA | NA | NR | NR | 1.05 (0.23) | 1.07 (0.37) |
| Diameter of FD (mean, SD) | 3.7 (0.7) | 4.0 (0.8) | NA | NA | 3.8 (0.7) | 4.0 (0.9) | NA | NA | NA | NA |
| Length of FD (mean, SD) | 17.0 (6.1) | 22.7 (8.4) | NA | NA | 18.3 (8.5) | 23.4 (6.6) | NA | NA | NA | NA |
| Adjunctive device | Coiling: 3 | Coiling: 2 | NA | NA | 0 | 0 | NA | NA | Coiling: 4 | Coiling: 50 |
| Use of antiplatelet, number | Clopidogrel: 30, ticagrelor: 46, prasugrel: 1, cangrelor: 9 | Clopidogrel: 15, ticagrelor: 18, prasugrel: 0, cangrelor: 0 | 285 | 84 | Aspirin + clopidogrel: 105 Aspirin + ticagrelor: 10 |
Aspirin + clopidogrel: 32 Aspirin + ticagrelor: 3 |
47 | 44 | NA | NA |
FRED: flow redirection endoluminal device; NA: not available; PED: pipeline embolization device.
Table 2.
Outcomes of patients included in studies.
| Variables/studies/study designs/study period/countries | Field et al. (2023) | Griessenauer et al. (2020) | El Naamani et al. (2023) | Pichardo et al. (2020) | Griessenauer et al. (2018) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Retrospective cohort | Retrospective cohort | Retrospective cohort | Retrospective cohort | Retrospective cohort | ||||||
| 2018–2022 | 2012–2019 | 2018–2021 | 2016–2019 | 2013–2017 | ||||||
| USA | USA | USA | Mexico | USA | ||||||
| Type of flow diverter | PED | FRED | PED | FRED | PED | FRED | PED | FRED | PED | FRED |
| Complete occlusion (6 months) | 38 (48%) | 16 (50%) | NA | NA | 65 (75%) | 17 (52%) | 43 (91%) | 40 (91%) | NA | NA |
| Complete occlusion (1 year) | 48 (60%) | 19 (59%) | NA | NA | NA | NA | 45 (96%) | 43 (98%) | NA | NA |
| Complete occlusion (last follow up) | 48 (60%) | 19 (59%) | NA | NA | 65 (75%) | 17 (52%) | 45 (96%) | 43 (98%) | 172 (78%) | 224 (79%) |
| Complete and near-complete occlusion (last follow up) | 48 (60%) | 19 (59%) | 220 (84%) | 71 (91%) | 65 (75%) | 17 (52%) | 45 (96%) | 43 (98%) | 192 (87%) | 274 (97%) |
| Follow up (mean, SD) | 20.6 (12.3) | 12.4 (7) | 14.0 (13.4) | 24.0 (18.1) | 8.8 (6.2) | 8.4 (7.6) | NA | NA | 10.3 (8.2) | 9.7 (4.5) |
| Favorable outcome (mRS ≤ 2) | NA | NA | 242 (85%) | 78 (94%) | 87 (100%) | 28 (100%) | 46 (98%) | 44 (100%) | 175 (97%) | 259 (99%) |
| Retreatment | 5 (6%) | 0 (0%) | 21/ (7%) | 6 (7%) | 7 (8%) | 1 (3%) | NR | NR | 10 | 0 |
| Total complications | 11 (14%) | 5 (16%) | 75 (26%) | 14 (17%) | 11 (10%) | 5 (14%) | 4 (9%) | 6 (14%) | 28 (14%) | 26 (10%) |
| Thromboembolic complications | 5 (6%) | 4 (12%) | 37 (13%) | 11 (13%) | NA | NA | 0 (0%) | 2 (5%) | 6 (3%) | 14 (5%) |
| Hemorrhagic complications | 2 (2%) | 0 (0%) | 14 (5%) | 3 (4%) | NA | NA | 1 (2%) | 0 (0%) | 3 (2%) | 2 (1%) |
| Parent artery stenosis/in-stent thrombosis | 4 (5%) | 1 (3%) | NA | NA | NA | NA | 0 (0%) | 1 (2%) | NA | NA |
FRED: flow redirection endoluminal device; mRS: modified Rankin scale; NA: not available; PED: pipeline embolization device.
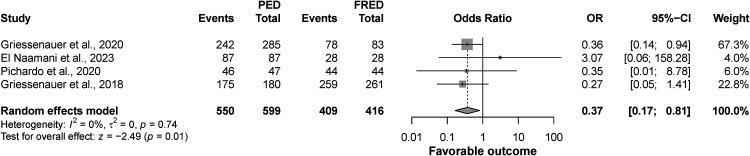
Outcome of favorable outcomes
The four studies included in this analysis showed that the rate of favorable outcomes was significantly higher in the FRED group compared to PED (OR: 0.37; CI: 0.17 to 0.81, p = 0.01), and there was no significant heterogeneity among the included studies (I2 = 0%, p = 0.74) (Figure 2).
Figure 2.
Forest plot for favorable outcome.
Outcome of complete/near complete occlusion at last follow up
The results from the analysis of five studies indicated that the complete/near complete occlusion at last follow up did not differ between PED and FRED groups (OR: 0.71; CI: 0.27 to 1.87, p = 0.49). However, there was a significant heterogeneity among the included studies (I2 = 81%, p < 0.01) (Figure 3).
Figure 3.
Forest plot for aneurysmal occlusion rates.
Outcome of complete occlusion at 6 months, 1 year, and at last follow up
The analysis performed on the complete occlusion at 6 months included three studies and revealed no significant difference between PED and FRED (OR: 1.46; CI: 0.66 to 3.21, p = 0.35), and there was no significant heterogeneity among the studies (I2 = 47%, p = 0.15). Similarly, analysis of two studies showed no significant difference between the two devices regarding complete occlusion at 1 year follow up (OR: 0.96; CI: 0.43 to 2.11, p = 0.91) (Figure 3).
The analysis was also performed on the complete occlusion at last follow up and included four studies. No significant difference was observed between PED and FRED groups (OR: 1.22; CI: 0.66 to 2.25, p = 0.52), and there was no significant heterogeneity among the studies (I2 = 49%, p = 0.12) (Figure 3).
Outcome of retreatment and follow up
The results from the analysis of four studies indicated that retreatment rates didn’t significantly differ between PED and FRED groups (OR: 2.79; CI: 0.7 to 11.11, p = 0.15), and there was no significant heterogeneity among the included studies (I2 = 45%, p = 0.14). In addition, there was no significant difference between the two groups in terms of follow-up duration (MD: −0.12; CI: −7.23 to 6.99, p = 0.97). However, a significant heterogeneity was observed (I2 = 92%, p < 0.01) (Figure 4).
Figure 4.
Forest plot for retreatment rates and follow up.
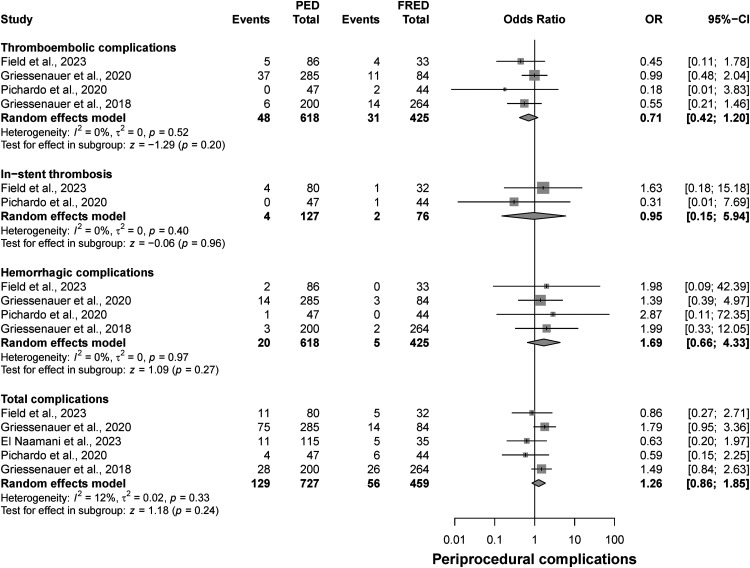
Outcome of thromboembolic, in-stent thrombosis, hemorrhagic, and total complications
The results from this analysis showed no significant differences between FRED and PED groups in terms of thromboembolic complications (OR: 0.71; CI: 0.42 to 1.2, p = 0.2), in-stent thrombosis (OR: 0.95; CI: 0.15 to 5.94, p = 0.96), hemorrhagic complications (OR: 1.69; CI: 0.66 to 4.33, p = 0.27), and total complications (OR: 1.26; CI: 0.86 to 1.85, p = 0.24) (Figure 5).
Figure 5.
Forest plot for periprocedural complications (thromboembolic, hemorrhagic, in-stent thrombosis, total complications).
Discussion
The present systematic review and meta-analysis aimed to compare between two flow diverters (PED and FRED). Our findings suggest no significant differences between the two devices in terms of complete or near complete occlusion rates, retreatment rates, follow-up duration, and total complications. However, a significant difference was observed between the two groups regarding functional outcomes with FRED device being more likely to achieve higher favorable outcome rate. All these findings were significant because it was shown that both devices could be used with similar effectiveness and safety in the treatment of intracranial aneurysms.
The structural variations between the FRED and PED devices imply that their effectiveness and outcomes in aneurysm occlusion might differ. The FRED, known for its more rigid and stable makeup, features a pore density <70% and a minimum porosity of 20 pores per square millimeter.26,27 In contrast, the PED exhibits a 70% density, but its performance shows a predictable parabolic variability. This variability is influenced by several factors, including the specific characteristics of the device, the diameter of the parent artery, and the curvature of the device. For instance, a slight oversizing of the PED can lead to increased porosity, which could potentially affect the effectiveness of aneurysm occlusion.28,29
However, the similarity in occlusion rates among these devices is quite expected. Prior data indicates that occlusion rates for FRED device vary between 33% and 88%.17,30–32 A study by Adeeb et al., 33 encompassing 465 aneurysms treated with PED, reported that complete occlusion (100%) was achieved in 78.2% of aneurysms, while near complete occlusion (90–99%) was observed in 7.6%, and partial occlusion (<90%) in 14.2% of cases.
While there were variations in the included studies regarding functional outcomes with some reporting no difference between the two devices and others do,3,22,24,25 our analysis revealed that FRED has a significantly more chances of achieving better functional outcomes when compared to PED.
Despite this, the study encompasses several limitations warranting consideration. One of the key limitations of this study is the restricted number of studies included, particularly those that meet the eligibility criteria. This limitation impacts the generalizability of the findings and may contribute to the observed heterogeneity in the results. In addition, the evidence presented in this study is from observational studies which may not be as cogent as that obtained from randomized controlled trials.
Conclusion
Our findings indicate that both devices (PED and FRED) demonstrate similar rates of complete or near-complete occlusion at the last follow up, as well as comparable rates of retreatment, follow-up duration, and total complications. However, a noteworthy distinction was observed in functional outcomes, with the FRED device showing a significantly higher likelihood of achieving higher favorable outcomes rate compared to the PED. Future studies with larger cohorts and longer follow-up periods are essential to further elucidate the long-term efficacy and safety of these flow diverters in the management of intracranial aneurysms.
Supplemental Material
Supplemental material, sj-docx-1-ine-10.1177_15910199241264345 for Comparison of pipeline embolization device and flow redirection endoluminal device in the treatment of intracranial aneurysms: A systematic review and meta-analysis by Basel Musmar, Atakan Orscelik, Hamza Salim, Fares Musmar, Nimer Adeeb, Kareem El Naamani, Muhammed Amir Essibayi, Samantha Spellicy, Jihad Abdelgadir, Adam A. Dmytriw, Aman B. Patel, Vitor Mendes Pereira, Hugo H. Cuellar-Saenz, Bharat Guthikonda, Ali Zomorodi, Pascal Jabbour and David Hasan in Interventional Neuroradiology
Footnotes
Authors’ contributions: BM, AO, FM, NA, KEN, MAE, SS, JA, AAD, ABP, VMP, HHC, BG, AZ, PJ, and DH contributed to the conception and design of the work, acquisition of data, and data analysis and interpretation, and drafted the work and revised it critically for important intellectual content. All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Availability of data and materials: The authors declare that all supporting data are available within the article.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Guarantor: AO
ORCID iDs: Basel Musmar https://orcid.org/0009-0000-4910-6090
Kareem El Naamani https://orcid.org/0000-0002-1054-9414
Muhammed Amir Essibayi https://orcid.org/0000-0001-8325-2382
Hugo H. Cuellar-Saenz https://orcid.org/0000-0002-8348-4535
Supplemental material: Supplemental material for this article is available online.
References
- 1.Musmar B, Adeeb N, Ansari J, et al. Endovascular management of hemorrhagic stroke. Biomedicines 2022; 10: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kallmes DF, Brinjikji W, Boccardi E, et al. Aneurysm study of pipeline in an observational registry (ASPIRe). Interv Neurol 2016; 5: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griessenauer CJ, Thomas AJ, Enriquez-Marulanda A, et al. Comparison of pipeline embolization device and flow re-direction endoluminal device flow diverters for internal carotid artery aneurysms: a propensity score-matched cohort study. Neurosurgery 2019; 85: E249–E255. [DOI] [PubMed] [Google Scholar]
- 4.Killer-Oberpfalzer M, Kocer N, Griessenauer CJ, et al. European multicenter study for the evaluation of a dual-layer flow-diverting stent for treatment of wide-neck intracranial aneurysms: the European flow-redirection intraluminal device study. AJNR Am J Neuroradiol 2018; 39: 841–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raymond J, Gentric JC, Darsaut TE, et al. Flow diversion in the treatment of aneurysms: a randomized care trial and registry. J Neurosurg 2017; 127: 454–462. [DOI] [PubMed] [Google Scholar]
- 6.Becske T, Potts MB, Shapiro M, et al. Pipeline for uncoilable or failed aneurysms: 3-year follow-up results. J Neurosurg 2017; 127: 81–88. [DOI] [PubMed] [Google Scholar]
- 7.Becske T, Kallmes DF, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 2013; 267: 858–868. [DOI] [PubMed] [Google Scholar]
- 8.Kallmes DF, Brinjikji W, Cekirge S, et al. Safety and efficacy of the pipeline embolization device for treatment of intracranial aneurysms: a pooled analysis of 3 large studies. J Neurosurg 2017; 127: 775–780. [DOI] [PubMed] [Google Scholar]
- 9.McDonald RJ, McDonald JS, Kallmes DF, et al. Periprocedural safety of pipeline therapy for unruptured cerebral aneurysms: analysis of 279 patients in a multihospital database. Interv Neuroradiol 2015; 21: 6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dagra A, Lucke-Wold B. Commentary: comparison of flow-redirection endoluminal device and pipeline embolization device in the treatment of intracerebral aneurysms. Neurosurgery 2023; 92: e3–e4. [DOI] [PubMed] [Google Scholar]
- 11.Dholakia RJ, Kappel AD, Pagano A, et al. In vitro angiographic comparison of the flow-diversion performance of five neurovascular stents. Interv Neuroradiol 2018; 24: 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White TG, Santhumayor BA, Turpin J, et al. Flow diverter surface modifications for aneurysm treatment: a review of the mechanisms and data behind existing technologies. Interv Neuroradiol Published online October 2023; 29: 15910199231207550. [DOI] [PubMed] [Google Scholar]
- 13.McDougall CG, Diaz O, Boulos A, et al. Safety and efficacy results of the flow redirection endoluminal device (FRED) stent system in the treatment of intracranial aneurysms: US pivotal trial. J Neurointerventional Surg 2022; 14: 577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kocer N, Islak C, Kizilkilic O, et al. Flow re-direction endoluminal device in treatment of cerebral aneurysms: initial experience with short-term follow-up results. J Neurosurg 2014; 120: 1158–1171. [DOI] [PubMed] [Google Scholar]
- 15.Möhlenbruch MA, Herweh C, Jestaedt L, et al. The FRED flow-diverter stent for intracranial aneurysms: clinical study to assess safety and efficacy. AJNR Am J Neuroradiol 2015; 36: 1155–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sayin B, Şenol YC, Daglioglu E, et al. Endovascular treatment of challenging aneurysms with FRED Jr flow diverter stents: a single-center experience. Jpn J Radiol 2022; 41: 322–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naamani K E, Saiegh FA, Chen CJ, et al. Treatment of cerebral aneurysms with the FRED Jr flow-diverting stent: a case series and meta-analysis. Clin Neurol Neurosurg 2022; 223: 107483. [DOI] [PubMed] [Google Scholar]
- 18.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ROBINS-I tool | Cochrane Methods. Accessed January 19, 2024. https://methods.cochrane.org/robins-i
- 20.Robins J, Breslow N, Greenland S. Estimators of the Mantel-Haenszel variance consistent in both sparse data and large-strata limiting models. Biometrics 1986; 42: 311–323. [PubMed] [Google Scholar]
- 21.Field NC, Custozzo A, Gajjar AA, et al. Comparison of pipeline embolization device, flow re-direction endoluminal device and surpass flow diverters in the treatment of intracerebral aneurysms. Interv Neuroradiol Published online August 27 2023: 15910199231196621. doi: 10.1177/15910199231196621 [DOI] [PubMed] [Google Scholar]
- 22.Griessenauer CJ, Enriquez-Marulanda A, Xiang S, et al. Comparison of PED and FRED flow diverters for posterior circulation aneurysms: a propensity score matched cohort study. J Neurointerventional Surg 2021; 13: 153–158. [DOI] [PubMed] [Google Scholar]
- 23.Naamani K E, Saad H, Chen CJ, et al. Comparison of flow-redirection endoluminal device and pipeline embolization device in the treatment of intracerebral aneurysms. Neurosurgery 2023; 92: 118–124. [DOI] [PubMed] [Google Scholar]
- 24.Griessenauer CJ, Thomas AJ, Enriquez-Marulanda A, et al. Comparison of pipeline embolization device and flow re-direction endoluminal device flow diverters for internal carotid artery aneurysms: a propensity score-matched cohort study. Neurosurgery 2019; 85: E249–E255. [DOI] [PubMed] [Google Scholar]
- 25.Pichardo O, Picazo A, Castillon O, et al. The Pipeline Endovascular Device versus the Flow Re-Direction Endoluminal Device for Cerebral Aneurysm. A One-Year Follow-up in a Single-Center Experience. 2021; 1(1). [Google Scholar]
- 26.Dmytriw AA, Phan K, Moore JM, et al. On flow diversion: the changing landscape of intracerebral aneurysm management. AJNR Am J Neuroradiol 2019; 40: 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuda Y, Chung J, Keigher Ket al. et al. A comparison between the new low-profile visualized intraluminal support (LVIS blue) stent and the flow redirection endoluminal device (FRED) in bench-top and cadaver studies. J Neurointerventional Surg 2018; 10: 274–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becske T, Brinjikji W, Potts MB, et al. Long-term clinical and angiographic outcomes following pipeline embolization device treatment of complex internal carotid artery aneurysms: five-year results of the pipeline for uncoilable or failed aneurysms trial. Neurosurgery 2017; 80: 40–48. [DOI] [PubMed] [Google Scholar]
- 29.Jou LD, Chintalapani G, Mawad ME. Metal coverage ratio of pipeline embolization device for treatment of unruptured aneurysms: reality check. Interv Neuroradiol 2016; 22: 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kocer N, Islak C, Kizilkilic O, et al. Flow re-direction endoluminal device in treatment of cerebral aneurysms: initial experience with short-term follow-up results. J Neurosurg 2014; 120: 1158–1171. [DOI] [PubMed] [Google Scholar]
- 31.Gan CL, Yang Z, Salahia G, et al. A single-centre experience and literature review of flow re-directional endoluminal device (FRED) in endovascular treatment of intracranial aneurysms. Clin Radiol 2021; 76: 238. e1-238.e8. [DOI] [PubMed] [Google Scholar]
- 32.Briganti F, Leone G, Ugga L, et al. Safety and efficacy of flow re-direction endoluminal device (FRED) in the treatment of cerebral aneurysms: a single center experience. Acta Neurochir 2016; 158: 1745–1755. [DOI] [PubMed] [Google Scholar]
- 33.Adeeb N, Moore JM, Wirtz M, et al. Predictors of incomplete occlusion following pipeline embolization of intracranial aneurysms: is it less effective in older patients? AJNR Am J Neuroradiol 2017; 38: 2295–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ine-10.1177_15910199241264345 for Comparison of pipeline embolization device and flow redirection endoluminal device in the treatment of intracranial aneurysms: A systematic review and meta-analysis by Basel Musmar, Atakan Orscelik, Hamza Salim, Fares Musmar, Nimer Adeeb, Kareem El Naamani, Muhammed Amir Essibayi, Samantha Spellicy, Jihad Abdelgadir, Adam A. Dmytriw, Aman B. Patel, Vitor Mendes Pereira, Hugo H. Cuellar-Saenz, Bharat Guthikonda, Ali Zomorodi, Pascal Jabbour and David Hasan in Interventional Neuroradiology