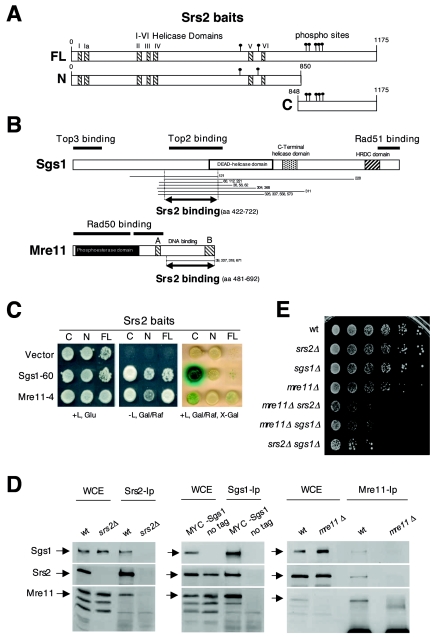
FIG. 1.
SRS2, SGS1, and MRE11 physically and genetically interact. (A) The protein regions corresponding to the three Srs2 baits used for the two-hybrid screening are shown: FL (full-length); N (N terminus); and C (C terminus). Consensus sites for Cdk1 kinase and the seven helicase domains (I and Ia to VI) are indicated by solid circles and striped boxes, respectively. (B) The protein fragments of Sgs1 and Mre11, identified by the two-hybrid analysis, are indicated by lines flanking the numbers of corresponding clones, under the schematic representation of the two proteins. Double arrowed lines indicate the Sgs1 and Mre11 protein regions involved in the interaction with Srs2. Previously mapped Sgs1 and Mre11 domains, involved in protein-protein interactions, are also shown. (C) An example of the two-hybrid interaction in haploid strains between Srs2 baits and Mre11-4 and Sgs1-60 clones is shown. The three bait strains, transformed with the corresponding prey plasmids or with pJG4-5 vector (CY5107, CY5109, CY5111, CY5448, CY5445, CY5442, CY5439, CY5436, and CY5435), were spotted on proper media with and without leucine (L), glucose (Glu), galactose (Gal), raffinose (Raf), and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). (D) Srs2, Sgs1, and Mre11 proteins were immunoprecipitated from whole-cell extracts (WCE) prepared from CY3262 and CY5680 or CY2715 and CY5683, respectively, as controls and then analyzed by Western blotting using specific antibodies. WCE lanes are a 1:10 dilution of the immunoprecipitated extracts. (E) The cellular growth of wild-type (W303), srs2Δ (CY2643), sgs1Δ (CY2570), mre11Δ (CY2730), mre11Δ srs2Δ (CY5492), mre11Δ sgs1Δ (CY5670), and srs2Δ sgs1Δ (CY3137) stains was evaluated by plating serial dilutions on YPD plates.