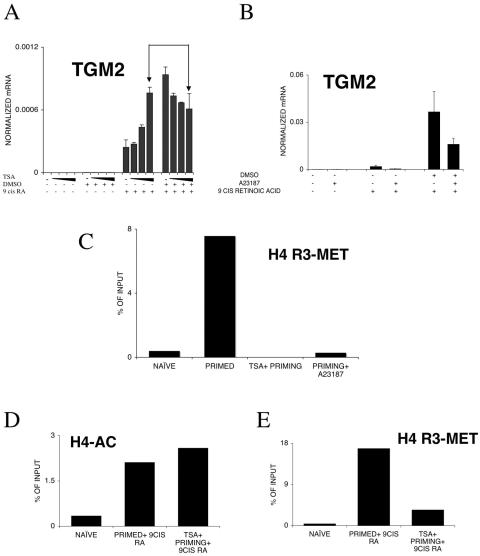
FIG. 8.
Modulation of TGM2 expression levels in HL-60 cells. (A) TSA pretreatment for 1 h of HL-60 cells enhances the retinoid response in naive cells (50, 100, and 150 nM TSA was used). TSA pretreatment for 1 h of HL-60 cells before DMSO priming reduces the effect of priming on TGM2 induction (50, 100, and 150 nM TSA was used). Arrows indicate the effect of pretreatment with 150 nM TSA on primed versus naive retinoid-treated cells. mRNA copy numbers were normalized to 36B4 transcript levels. Values are the means of three independent QPCR measurements ± standard deviations. (B) Activation of PAD4 after priming by a 15-min treatment with 1 μM calcium ionophore A23187 reduces the priming effect. mRNA copy numbers were normalized to cyclophilin transcript levels. Values are the means of three independent QPCR measurements ± standard deviations. (C) H4R3 methylation levels are changed during priming and modulated by pretreatment with 100 nM TSA for 1 hour or a 15-min treatment with 1 μM calcium ionophore A23187. H4R3 methylation was determined by chromatin immunoprecipitation on the HR1 enhancer element. Copy numbers are expressed as percentages of the input. (D) H4 acetylation levels in primed, retinoid-treated cells and TSA-pretreated, primed retinoid-treated cells. Cells were pretreated with 100 nM TSA for 1 hour, and H4 acetylation levels were determined by chromatin immunoprecipitation on the HR1 enhancer element. Copy numbers are expressed as percentages of the input. (E) H4R3 methylation levels in primed, retinoid-treated cells and TSA-pretreated, primed retinoid-treated cells. Cells were pretreated with 100 nM TSA for 1 hour, and H4R3 methylation levels were determined by chromatin immunoprecipitation on the HR1 enhancer element. Copy numbers are expressed as percentages of the input. The values of the no-antibody controls were subtracted. Values are the means of three independent QPCR measurements of a representative experiment. Chromatin immunoprecipitation results were confirmed in two independent chromatin preps with two independent immunoprecipitations, respectively. +, present; −, absent.