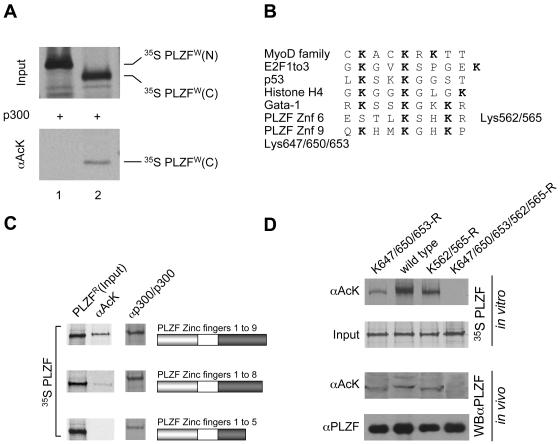
FIG. 3.
PLZF is acetylated in the zinc finger DNA binding region. (A) N- and C-terminal parts of PLZF (amino acids 1 to 455 and 455 to 673, respectively) were expressed and labeled in vitro with [35S]methionine using the wheat germ translation system. In vitro-translated proteins were acetylated in the presence of acetyl-CoA and p300. The degree of acetylation was evaluated by immunoprecipitation with anti-acetyl lysine (αAcK) antibodies (lower panel). (B) Alignment of previously described acetylation sites in other proteins and the potential PLZF sites. (C) Mapping of PLZF acetylation sites. Deletion mutants of PLZF were translated in vitro using rabbit reticulocyte lysate and [35S]methionine and were tested for acetylation (PLZFR) by immunoprecipitation with anti-acetyl-lysine antibodies (αAcK). Corresponding deletion mutants are represented by schematics in which the grey box represents the POZ domain of PLZF and the dark box represents its zinc finger region. The input of each PLZF protein is shown in the column labeled PLZFR. Interactions of PLZF proteins with p300 were tested in vitro by immunoprecipitation using p300 protein immobilized on a protein A/G matrix with a p300 polyclonal antibody (αp300/p300) as bait. (D) PLZF lysine mutants assessed for acetylation in vitro and in vivo. Lysine (K) residues of interest were changed to arginines (R). A mutant lacking the zinc finger 9 acetylation site (K647/650/653-R), a mutant lacking the zinc finger 6 acetylation site (K562/565-R), and the double mutant (K647/650/653/562/565-R) were compared to wild-type PLZF (wild type). In vitro and in vivo experiments were performed with in vitro 35S-labeled PLZF proteins and transfected 293T cell extracts, respectively. The acetylation level of PLZF was assessed by immunoprecipitation of 35S-labeled PLZF with an anti-acetyl-lysine antibody (αAcK). Polyclonal anti-PLZF antibody (αPLZF) was used to immunoprecipitate the total protein (input). For in vivo analysis, proteins were immunoprecipitated as above, and immunoprecipitates were subjected to Western blot analysis with monoclonal anti-PLZF antibodies (WBαPLZF). Enhanced chemiluminescence was used for detection.