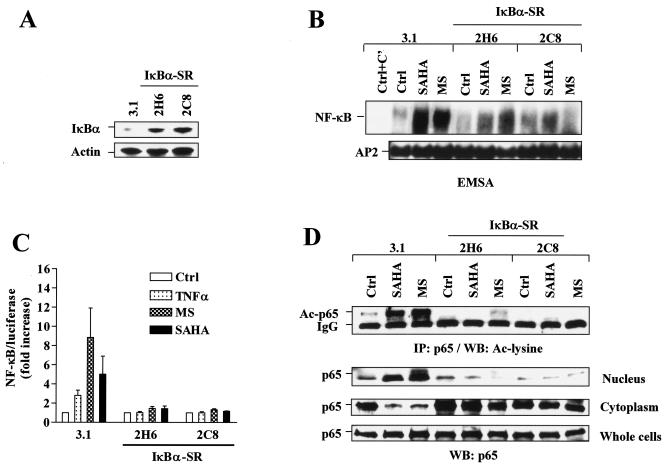
FIG. 3.
HDAC inhibitors fail to induce NF-κB activation, as well as RelA/p65 acetylation and nuclear translocation in IκBα-SR-transfected cells. (A) U937 cells were stably transfected with Ser32/Ser36-mutated IκBα cDNA (IκBα-SR) or an empty vector (pcDNA3.1). A Western blot analysis was performed to monitor expression of IκBα. (B) Two separate clones (2H6 and 2C8) of IκBα-SR/U937 cells as well as control 3.1/U937 cells were treated for 24 h with 2 μM of either MS-275 or SAHA, after which nuclear extracts were prepared and subjected to EMSA. AP2/DNA binding was assessed by using an AP2 consensus oligonucleotide for equal loading controls. Ctrl+C', nuclear extract of untreated 3.1/U937 cells preincubated with unlabeled oligonucleotides as specific competitor (C′) controls. Two additional studies yielded equivalent results. (C) IκBα-SR/U937 and 3.1/U937 cells were transfected with NF-κB/Luc and then exposed to 2 μM of MS-275 or SAHA for 24 h, after which a luciferase assay was performed to monitor NF-κB activity. In parallel, cells were treated with 10 ng/ml TNF-α for 30 min as a control. Relative luciferase activities were determined by normalizing to total protein. For each cell line, values for untreated controls were arbitrarily set to a value of 1, and NF-κB/luciferase activity is expressed as the fold increase relative to untreated controls (ctrl). The results represent the means ± SD for three separate experiments performed in triplicate. (D) IκBα-SR/U937 and 3.1/U937 cells were treated as described for panel B, after which immunoprecipitation (IP) and a Western blot (WB) analysis were performed to evaluate the acetylation (Ac) status (upper panel) and subcellular distribution of RelA/p65 (lower panels), respectively. Ctrl, control. The results are representative of three separate experiments. For Western blot analyses in panels A and D, each lane was loaded with 30 μg of protein; blots were reprobed with anti-β-actin, as indicated, to ensure equivalent loading and transfer of protein.