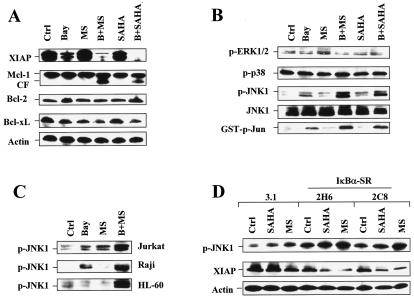
FIG. 7.
NF-κB inhibition results in JNK1 activation and XIAP downregulation in HDAC inhibitor-treated cells. Ctrl, control. (A and B) U937 cells were exposed for 24 h to 3 μM Bay 11-7082 with (B) or without (Bay) 2 μM MS-275 (MS) or 1 μM SAHA, after which a Western blot analysis was performed to evaluate alterations in expression of antiapoptotic proteins (A) and phosphorylation (activation) of ERK1/2, p38, and JNK (B). CF, cleavage fragment. (B) Alternatively, kinase assays were performed, as described in Materials and Methods, to assess SAPK/JNK activity, reflected by phosphorylated (p) GST-c-Jun (bottom panel). (C) Jurkat, Raji, and HL-60 cells were exposed to 2 μM MS-275 with (B+MS) or without (MS) Bay 11-7082 (Jurkat, 2 μM; Raji, 5 μM; HL-60, 3 μM), after which cells were lysed and subjected to Western blot analysis to monitor phosphorylation of JNK. Bay, Bay 11-7082; p, phosphorylated. (D) IκBα-SR/U937 cells, as well as 3.1/U937 cells, were treated for 24 h with 2 μM of either MS-275 (MS) or SAHA, after which a Western blot analysis was performed to assess JNK phosphorylation and XIAP expression. p, phosphorylated. For Western blot analysis in panels A through D, each lane was loaded with 30 μg of protein; blots were subsequently stripped and reprobed for the expression of β-actin, as indicated, to ensure equivalent loading and transfer of protein. The results are representative of three separate experiments.