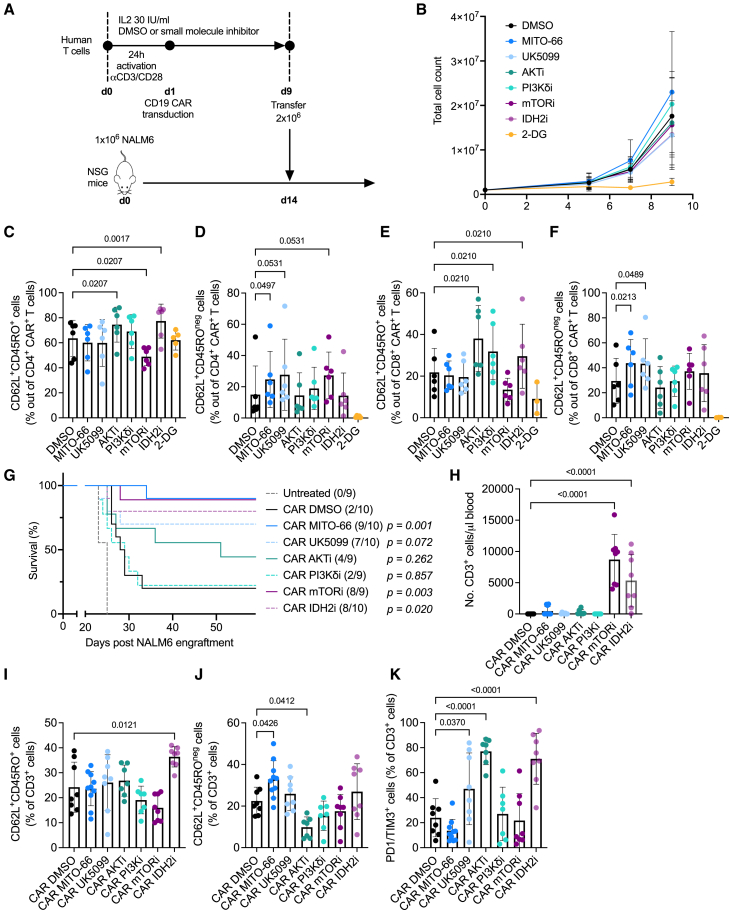
Figure 5.
Benchmarking MITO-66 against other small molecules influencing memory differentiation
(A) Experimental scheme depicting CD19-CAR T cell generation in the presence of different small-molecule inhibitors (MITO-66 and UK-5099 [25 μM], AKT-VIII [AKTi, 1 μM], idelalisib [PI3KδI, 200 nM], rapamycin [mTORi, 100 nM], enasidenib [IDH2i, 5 μM], and 2-deoxyglucose [2-DG, 2 mM], molecule concentrations were selected based on previous literature). (B) T cell expansion measured by machine-assisted trypan blue-based cell counting at day 5, 7, and 9 post-activation. (C–F) CD62L/CD45RO double-positive cells out of CD4 (C) or CD8 (E) CAR T cells or CD62L-positive, CD45RO-negative stem cell-like memory T cells out of CD4 (D) or CD8 (F) CAR T cells at day 9 post-activation. (B–F) Six donors, pooled data from four independent experiments. (G) Survival of mice receiving no treatment or inhibitor-conditioned CAR T cells. Surviving mice out of total treated mice are indicated in brackets. p values indicate statistical difference versus CAR DMSO. (H) Number (No.) of transferred CD3 T cells in the blood of mice analyzed by flow cytometry at day 11 post-ACT. (I–K) Percentage of CD62L/CD45RO double-positive cells (I), CD62L-positive, CD45RO-negative cells (J), or PD1/TIM3 double-positive cells (K) out of transferred CD3 T cells at day 11 post-ACT. (G–K) Three human donors into 9–10 total mice, pooled data from 2 independent experiments, only flow cytometry data with >20 events were used for phenotypic analysis. Data are represented as mean ± SD. Statistics are based on one-way ANOVA (C–F and H–K) or log rank test (G). See also Figure S3.