Abstract
Pathological scars, including hypertrophic scar and keloid are the result of excessive tissue repair and are influenced by biomechanical forces like tension, mechanical pressure, and stiffness. These forces significantly impact scar development and progression, affecting wound healing, collagen deposition, and tissue remodeling. Understanding how these mechanical stimuli contribute to scar development is essential for devising effective therapeutic interventions. Clinically, reducing wound tension and applying mechanical pressure are key strategies for managing pathological scars. Techniques like super-tension-reduction suturing, stress-shielding polymers, and force-modulating tissue bridges (FMTB) have been shown to effectively alleviate tension and reduce scar proliferation. Additionally, Pressure Garment Therapy (PGT) is widely used to treat hypertrophic scars by reducing tissue stiffness, limiting collagen buildup, and promoting collagen realignment. Despite challenges such as discomfort and uneven pressure application, ongoing research focuses on enhancing these therapies through mechanosensitive technologies to improve both efficacy and patient comfort. This review highlights the role of biomechanical forces in scar formation and discusses therapeutic approaches that target these forces to improve clinical outcomes.
Keywords: pathological scars, biomechanical forces, hypertrophic scar, keloid, tissue remodeling
Introduction
Pathological scars are a type of fibrotic skin disorder that result from excessive tissue repair following trauma.1 Despite the prevalence of this condition, there are currently no specific treatments available. Pathological scars are often associated with symptoms such as itching and burning pain, while the contracture and tension caused by hypertrophic scars can lead to joint deformities and functional limitations, severely impairing the quality of life for affected individuals.2 Notably, approximately 70% of burn patients develop scar proliferation, highlighting the significant clinical burden of pathological scars.3
The formation and progression of pathological scars are strongly influenced by biomechanical forces. External mechanical stimuli, including tension, mechanical pressure, and stiffness, play crucial roles in the development and properties of scar tissue.4–6 Mechanobiological effects extend across various biological scales, from molecular signaling to cellular behavior and tissue architecture.7 Among these, local wound tension has been identified as a key modulator of wound healing and scar formation.8,9 Clinical evidence suggests that reducing local wound tension is an effective strategy for minimizing pathological scar development, and it has been widely adopted as a preventive approach.10,11
Mechanical pressure also plays a pivotal role in managing pathological scars. Pressure garment therapy (PGT) has been a cornerstone of treatment since the 1970s, especially for burn patients.12 By applying controlled pressure, PGT reduces scar thickness, increases pliability, and curbs collagen deposition, thereby improving scar outcomes.13 This mechanical force alters the local microenvironment by reducing blood flow, limiting nutrient availability, and promoting scar tissue remodeling.12,14
In addition to tension and pressure, stiffness—the tissue’s resistance to deformation—is another critical parameter in both the assessment and treatment of pathological scars.15 Higher stiffness correlates with more severe scars, characterized by increased collagen density and reduced elasticity. Monitoring changes in tissue stiffness over time provides valuable insight into scar maturation and helps clinicians evaluate therapeutic efficacy.16
This review will explore the distinct and interconnected roles of tension, mechanical pressure, and stiffness in the formation and treatment of pathological scars. By examining the mechanobiological effects of these forces, we aim to provide a comprehensive understanding of their impact on scar pathophysiology and inform the development of more targeted therapeutic strategies.
Review
This literature review was conducted using the PubMed database, focusing on articles published over the past ten years (2013–2023). The search targeted studies related to hypertrophic scars, keloids, and pathological scars, specifically examining the roles of tension, mechanical pressure, and stiffness in scar formation and treatment. Additionally, literature on the clinical applications of mechanical force interventions—such as tension-shielding techniques and the use of compressive garments—was included to assess their efficacy in scar prevention and management through mechanobiological effects.
Inclusion criteria for this review were restricted to clinical trials, systematic reviews, and meta-analyses that investigated scar pathology and treatment methods in human subjects. Studies were excluded if they focused solely on animal models, lacked relevance to clinical applications in scar management, or did not directly address the influence of mechanical forces on pathological scarring.
The Influence of Mechanobiological Effects on Pathological Scar Formation
Wound healing occurs within a dynamic and complex mechanical microenvironment, where various mechanical stimuli influence key cellular processes such as proliferation, differentiation, and migration.17 These stimuli play an essential role in determining tissue homeostasis and the outcome of repair. Mechanical signals—such as tension, stiffness, and pressure—interact and regulate one another at physiological levels, exerting profound effects on scar formation.7 The interplay between these forces is critical for guiding cellular behavior during the healing process, ultimately influencing the development and progression of pathological scars.
Tension
Increased local tension resulting from wound defects, along with the location of high tension at specific body sites, plays a crucial role in the onset of pathological scars.
Wound defects that lead to elevated local tension prolong and intensify the inflammatory response within the dermal reticular layer.9 This repeated inflammation contributes to an abnormal proliferation of blood vessels, which in turn extends the inflammatory phase of wound healing. The excessive release of inflammatory cells and cytokines causes dysfunction in fibroblasts, ultimately triggering the formation of pathological scars.
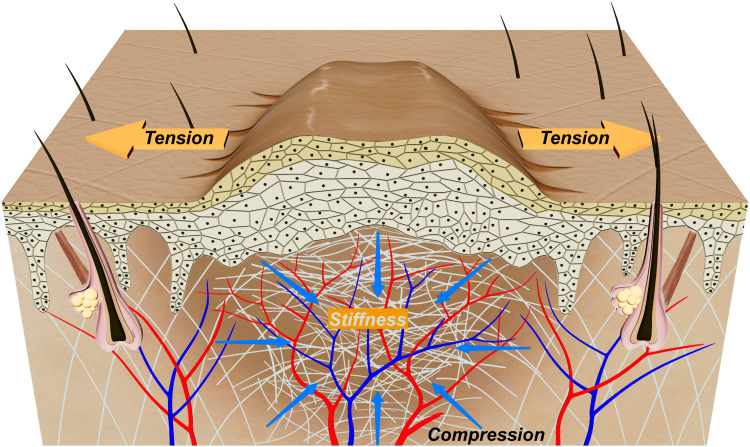
Furthermore, high tension at specific wound sites, such as the anterior chest and back, is a significant contributor to pathological scarring. Areas subject to greater mechanical stress are more prone to pathological scarring.18 Studies measuring tissue tension around scar nodules have shown that tension is higher at the edges of the nodules and lower at the center. This suggests that keloids tend to develop a dumbbell-like shape, with excessive growth at both ends due to elevated tension in these regions. (Figure 1).9 Digital modeling of local tissue tension supports this observation, showing higher tension in all directions around areas with dumbbell or crab-like changes.19
Figure 1.
Tension, Continuous Pressure, and Stiffness in Hypertrophic Scar Skin.
Animal models have been extensively used to study the effects of increased local tension on hypertrophic scar formation.20 In particular, models using metal rings to apply sustained tension on mouse tail wounds have closely replicated the histopathological features of human hypertrophic scars.21 For example, a metal ring with a 2 cm diameter produces a higher tension environment, leading to more pronounced scar proliferation and longer-lasting scars, which closely resemble human scar tissue. This approach overcomes some limitations of previous animal models, such as those involving rabbit ears and Duroc pigs, and provides a more reliable source of antibodies for molecular biology studies. Additionally, the development of gene knockout models has become more feasible using these tension-induced scar models.
In summary, local wound tension plays a pivotal role in the formation and progression of pathological scars by prolonging the inflammatory response and contributing to abnormal scar tissue growth. Clinical evidence and animal models consistently demonstrate that increased tension leads to more severe scar proliferation, highlighting the importance of managing tension to prevent pathological scarring. Understanding how mechanical tension influences cellular processes provides a foundation for developing targeted therapeutic strategies aimed at mitigating its effects.
Stiffness
While tension exerts a significant influence on pathological scar formation, stiffness—the resistance of tissue to deformation—plays an equally crucial role in determining scar severity and progression. As scars form and mature, the accumulation and disorganization of collagen fibers lead to increased tissue stiffness, a hallmark of pathological scarring.22 The deposition of a stiff extracellular matrix (ECM) is a defining feature of pathological scars, where abnormal collagen accumulation and disorganized tissue structure lead to increased stiffness and prolonged scar rigidity, contributing to impaired tissue function.23
Tissue stiffness directly impacts cellular behavior, influencing processes such as cell proliferation, differentiation.5 One critical aspect is the role of mechanical stiffness in activating fibroblasts, which promotes their transformation into myofibroblasts. This differentiation is linked to increased collagen deposition, contributing to the excessive scarring seen in hypertrophic scars.24 Researchers have identified key signaling pathways, such as TGF-β/Smad and FAK, that are activated in response to ECM stiffness.25 These pathways not only promote fibroblast activation but also enhance the contractility of myofibroblasts, exacerbating scar tissue formation. These findings suggest that modulating ECM stiffness or targeting key mechanotransduction pathways may provide therapeutic avenues to control fibroblast activation and limit scar formation.
In summary, stiffness plays a crucial role in the progression of pathological scars by regulating fibroblast activity and scar tissue rigidity. Modulating the mechanical environment offers promising avenues for therapeutic interventions aimed at reducing excessive scar formation and promoting healthy tissue remodeling.
Mechanical Pressure
Mechanical pressure in the formation of pathological scars arises from several critical factors.26 During the development of pathological scars, the internal mechanical forces can be primarily categorized into two main components: skin tension and wound contraction, and fibroblast contractility.27 These forces play a significant role in both wound healing and scar hypertrophy, making their study as important as other types of mechanical influences.
Skin Tension and Wound Contraction: As the wound heals, skin tension applies mechanical stress to the scar tissue. This tension is caused by the surrounding skin as it attempts to close the wound.28 As the scar tissue expands, it is subjected to mechanical forces from the surrounding skin, contributing to the overall stress and tension within the scar.29 Myofibroblasts drive wound contraction, pulling the wound edges together and creating additional internal mechanical pressure as the tissue contracts.
Fibroblast Contractility: Fibroblasts, particularly myofibroblasts, contract the extracellular matrix (ECM) as they respond to signals like transforming growth factor-beta (TGF-β) and mechanical stress.30 Myofibroblasts play a pivotal role in wound healing and pathological fibrosis by remodeling the ECM and stiffening the tissue.31 These cells generate considerable traction forces within the collagen network, further increasing mechanical pressure within the scar.
Positive Feedback Loop: As the ECM becomes stiffer, it further stimulates fibroblasts and myofibroblasts, perpetuating their contractile activity.32 This feedback loop perpetuates the mechanical pressure buildup, driving ongoing fibrosis and scar formation.25
Utilizing Mechanobiological Effects to Prevent and Treat Pathological Scars
In clinical practice, the biomechanical effects of mechanical forces play a crucial role in the prevention and treatment of pathological scars. Two key strategies have emerged: reducing tension at wound sites and utilizing mechanical pressure to suppress scar formation and progression. By leveraging these mechanobiological principles, clinicians aim to effectively manage and mitigate the risk of hypertrophic and keloid scar development.
Reducing Wound Tension
Reducing tension, or local wound tension, is one of the most effective ways to prevent the development of pathological scars. Clinical studies consistently show that areas of high tension, such as the anterior chest and back, are more susceptible to hypertrophic scar formation.33 To combat this, various techniques have been developed to shield the wound from excessive tension, including the use of advanced suturing materials and techniques that minimize stress at the incision site.34
For surgically removable hypertrophic scars, keloids, and surgical incision scars, prevention efforts primarily focus on suppressing local tension at the incision site. Clinical studies have consistently shown that shielding incision tension can significantly reduce the formation of post-surgical scars, particularly in high-tension areas where scar inhibition is more effective.35 With advancements in medical technology and modern suture materials, the goals for new suture development now include predictable tensile strength, superior knotting performance, and the ability to carry antimicrobial agents to prevent infection.36
Current methods for reducing incision tension include Subcutaneous Super-Tension-Reduction Suturing, Stress-Shielding Polymers, and Force-Modulating Tissue Bridges (FMTB), all of which have demonstrated satisfactory results in clinical practice.35,37,38 (Figure 2) The key techniques in Subcutaneous Super-Tension-Reduction Suturing involve: wedge-shaped excision of superficial fascia tissue approximately 2 cm from the wound edge, placing sutures at 10 mm intervals about 10 mm from the wound margin on each side, elevating the deep dense fibrofatty tissue along with the loose superficial fat to create an “ultrathin flap”, and removing excess fat during suturing.39 These four technical steps collectively reduce incision tension, ensure blood supply to the wound area, and promote wound healing. Stress-Shielding Polymers help mitigate the skin’s natural tension and shield the wound from tension generated by bodily movements during healing, thus stabilizing the surrounding tissues and preventing excessive scarring.40 Similarly, FMTB protects the wound from localized tension during healing by absorbing the tensile forces from deeper tissue layers beneath the dermis. This shielding reduces tissue necrosis caused by suture pressure and lowers the overall tensile forces exerted on the wound, effectively improving blood perfusion at the wound edges and promoting healing.41
Figure 2.
Current Clinical Approaches for Reducing Wound Tension to Mitigate Hypertrophic Scar Formation. (A). Subcutaneous Super-Tension-Reduction Suturing; (B) Stress-Shielding Polymers; (C) Force-Modulating Tissue Bridges (FMTB).
Research has also indicated that another important factor in keloid formation is the transformation of normal dermal stem cells into specialized keloid stem cells or progenitor cells. Contamination of the surgical site by surrounding normal dermal stem cells can diminish the effectiveness of tension-reducing sutures, leading to keloid recurrence.42 Therefore, a combination of surgical excision, tension-reducing sutures, and postoperative radiotherapy (within 24 hours, 15 Gy-20 Gy) is currently recommended for the treatment of pathological scars, yielding satisfactory outcomes.37
Mechanical Pressure Therapy to Inhibit Hypertrophic Scars
Pressure Garment Therapy (PGT) is currently the standard method for preventing and treating hypertrophic scars, particularly following burns. This approach is especially useful in cases of extensive burns, where surgical removal is not feasible. PGT has become a widely recognized, simple, and cost-effective first-line option for both the prevention and treatment of pathological scars.43
PGT has been shown to be effective in reducing scar height, enhancing scar elasticity, and alleviating symptoms such as redness and itching.44 Clinical studies, including four randomized controlled trials, have confirmed the efficacy of PGT.45 The therapy works by applying continuous mechanical pressure to compress blood vessels within the scar tissue, which reduces the supply of nutrients and oxygen, inhibits collagen deposition, and promotes the realignment of collagen fibers. These effects collectively contribute to the reduction of scar proliferation and, importantly, the decrease in tissue stiffness.46,47
However, PGT is not without its challenges. Common drawbacks include discomfort during wear, heat retention, abnormal sensations, and the need for prolonged use to achieve noticeable results. Additionally, pressure decay over time requires frequent garment replacement, and uneven pressure application at joint areas further reduces the therapy’s effectiveness. Efforts to address these issues, such as the use of pressure pads to improve pressure distribution at joints, have led to increased discomfort and inconvenience without fundamentally resolving the problem.48 As a result, current research is focused on the development of mechanosensitive sensors and molecular and cellular interventions aimed at enhancing mechanosensitivity. These advancements seek to improve the precision, comfort, and overall efficacy of pressure therapy, including its ability to reduce tissue stiffness, making it a more convenient and effective treatment option for hypertrophic scars.
Conclusion
In conclusion, biomechanical forces such as tension, mechanical pressure, and stiffness are key factors influencing the development and treatment of pathological scars. Clinical strategies targeting these mechanical factors, including the reduction of wound tension and the application of mechanical pressure through therapies like PGT, have demonstrated significant efficacy in reducing scar proliferation and improving outcomes for patients with hypertrophic scars. Reducing wound tension is particularly effective in preventing scar formation, while PGT remains a cornerstone in scar treatment by reducing tissue stiffness and promoting collagen realignment.
This review highlights the critical role of mechanobiological effects in scar pathophysiology and provides a comprehensive overview of current clinical approaches that leverage these forces for therapeutic benefit. By understanding the interactions between mechanical stimuli and scar formation, we can develop more targeted interventions to manage pathological scars more effectively. The insights provided in this review will contribute to improving treatment strategies, guiding future research, and enhancing patient care in the field of scar management.
Funding Statement
This work was supported by the Shandong Provincial Natural Science Foundation Youth Program (ZR2023QH266).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113–125. doi: 10.2119/molmed.2009.00153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiseman J, Ware RS, Simons M, et al. Effectiveness of topical silicone gel and pressure garment therapy for burn scar prevention and management in children: a randomized controlled trial. Clin Rehabil. 2020;34(1):120–131. doi: 10.1177/0269215519877516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finnerty CC, Jeschke MG, Branski LK, et al. Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet. 2016;388(10052):1427–1436. doi: 10.1016/S0140-6736(16)31406-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muller B, Mazza E, Schiestl C, Elrod J. Longitudinal monitoring and prediction of long-term outcome of scar stiffness on pediatric patients. Burns Trauma. 2021;9:tkab028. doi: 10.1089/wound.2013.0485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosseini M, Brown J, Khosrotehrani K, Bayat A, Shafiee A. Skin biomechanics: a potential therapeutic intervention target to reduce scarring. Burns Trauma. 2022;10:tkac036. doi: 10.1093/burnst/tkac036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin J, Zhang S, Yang C, et al. Mechanotransduction in skin wound healing and scar formation: potential therapeutic targets for controlling hypertrophic scarring. Front Immunol. 2022;13:1028410. doi: 10.1097/00004630-199007000-00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15(12):802–812. doi: 10.1038/nrm3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verhaegen PDHM, Van Zuijlen PPM, Pennings NM, et al. Differences in collagen architecture between keloid, hypertrophic scar, normotrophic scar, and normal skin: an objective histopathological analysis. Wound Repair Regen. 2009;17(5):649–656. doi: 10.1111/j.1524-475X.2009.00533.x [DOI] [PubMed] [Google Scholar]
- 9.Ogawa R, Akaishi S. Endothelial dysfunction may play a key role in keloid and hypertrophic scar pathogenesis - Keloids and hypertrophic scars may be vascular disorders. Med Hypotheses. 2016;96:51–60. doi: 10.1016/j.mehy.2016.09.024 [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Mo Y, Chen Y, et al. Application and effect of tension-reducing suture in surgical treatment of hypertrophic scar. BMC Surg. 2024;24(1):119. doi: 10.1186/s12893-024-02390-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trace AP, Enos CW, Mantel A, Harvey VM. Keloids and hypertrophic scars: a spectrum of clinical challenges. Am J Clin Dermatol. 2016;17:201–223. doi: 10.1007/s40257-016-0175-7 [DOI] [PubMed] [Google Scholar]
- 12.Li M, Wang P, Li J, et al. NRP1 transduces mechanical stress inhibition via LATS1/YAP in hypertrophic scars. Cell Death Discov. 2023;9(1):341. doi: 10.1038/s41420-023-01635-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larson DL, Abston S, Evans EB, Dobrkovsky M, Linares HA. Techniques for decreasing scar formation and contractures in the burned patient. J Trauma. 1971;11(10):807–823. doi: 10.1097/00005373-197110000-00001 [DOI] [PubMed] [Google Scholar]
- 14.DeBruler DM, Baumann ME, Blackstone BN, et al. Role of early application of pressure garments following burn injury and autografting. Plast Reconst Surg. 2019;143(2):310e–321e. doi: 10.1097/PRS.0000000000005270 [DOI] [PubMed] [Google Scholar]
- 15.Oliveira GV, Chinkes D, Mitchell C, et al. Objective assessment of burn scar vascularity, erythema, pliability, thickness, and planimetry. Dermatol Surg. 2005;31:48–58. doi: 10.1111/j.1524-4725.2005.31004 [DOI] [PubMed] [Google Scholar]
- 16.Clark JA, Cheng JC, Leung KS. Mechanical properties of normal skin and hypertrophic scars. Burns. 1996;22:443–446. doi: 10.1016/0305-4179(96)00038-1 [DOI] [PubMed] [Google Scholar]
- 17.Chu S-Y, Chou C-H, Huang H-D, et al. Mechanical stretch induces hair regeneration through the alternative activation of macrophages. Nat Commun. 2019;10(1):1524. doi: 10.1038/s41467-019-09402-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomtschik J, Anand N, Bustos SS, Martinez-Jorge J, Wyles SP. Practical management of hypertrophic scarring: the mayo clinic experience. Archives of Dermatological Res. 2024;316(2):77. doi: 10.1007/s00403-023-02802-3 [DOI] [PubMed] [Google Scholar]
- 19.Akaishi S, Akimoto M, Ogawa R, Hyakusoku H. The relationship between keloid growth pattern and stretching tension: visual analysis using the finite element method. Ann Plast Surg. 2008;60:445–451. doi: 10.1097/SAP.0b013e3181238dd7 [DOI] [PubMed] [Google Scholar]
- 20.Mascharak S, desJardins-Park HE, Davitt MF, et al. Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science. 2021;372(6540). doi: 10.1126/science.aba2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou S, Wang W, Zhou S, et al. A novel model for cutaneous wound healing and scarring in the rat. Plast Reconst Surg. 2019;143(2):468–477. doi: 10.1097/PRS.0000000000005274 [DOI] [PubMed] [Google Scholar]
- 22.Corr DT, Hart DA. Biomechanics of scar tissue and uninjured skin. Adv Wound Care. 2013;2(2):37–43. doi: 10.1089/wound.2011.0321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walraven M, Hinz B. Therapeutic approaches to control tissue repair and fibrosis: extracellular matrix as a game changer. Matrix Biol. 2018;71–72:205–224. doi: 10.1016/j.matbio.2018.02.020 [DOI] [PubMed] [Google Scholar]
- 24.Berry CE, Downer M, Morgan AG, et al. The effects of mechanical force on fibroblast behavior in cutaneous injury. Front Surg. 2023;10:1167067. doi: 10.3389/fsurg.2023.1167067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Younesi FS, Miller AE, Barker TH, Rossi FMV, Hinz B. Fibroblast and myofibroblast activation in normal tissue repair and fibrosis. Nat Rev Mol Cell Biol. 2024;25:617–638. doi: 10.1038/s41580-024-00716-0 [DOI] [PubMed] [Google Scholar]
- 26.Fernandes MG, da Silva LP, Cerqueira MT, et al. Mechanomodulatory biomaterials prospects in scar prevention and treatment. Acta Biomater. 2022;150:22–33. doi: 10.1016/j.actbio.2022.07.042 [DOI] [PubMed] [Google Scholar]
- 27.Seo BR, Chen X, Ling L, et al. Collagen microarchitecture mechanically controls myofibroblast differentiation. Proc Natl Acad Sci USA. 2020;117(21):11387–11398. doi: 10.1073/pnas.1919394117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnes LA, Marshall CD, Leavitt T, et al. Mechanical forces in cutaneous wound healing: emerging therapies to minimize scar formation. Advances in Wound Care. 2018;7(2):47–56. doi: 10.1089/wound.2016.0709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karppinen SM, Heljasvaara R, Gullberg D, Tasanen K, Pihlajaniemi T. Toward understanding scarless skin wound healing and pathological scarring. F1000Res. 2019;8:787. doi: 10.12688/f1000research.18293.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue M, Jackson CJ. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care. 2015;4(3):119–136. doi: 10.1089/wound.2013.0485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nature Reviews Molecular Cell Biology. 2002;3(5):349–363. doi: 10.1038/nrm809 [DOI] [PubMed] [Google Scholar]
- 32.Hall MS, Alisafaei F, Ban E, et al. Fibrous nonlinear elasticity enables positive mechanical feedback between cells and ECMs. Proc Natl Acad Sci USA. 2016;113(49):14043–14048. doi: 10.1073/pnas.1613058113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mustoe TA, Cooter RD, Gold MH, et al. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110(2):560–571. doi: 10.1097/00006534-200208000-00031 [DOI] [PubMed] [Google Scholar]
- 34.Ogawa R, Mitsuhashi K, Hyakusoku H, Miyashita T. Postoperative electron-beam irradiation therapy for keloids and hypertrophic scars: retrospective study of 147 cases followed for more than 18 months. Plast Reconst Surg. 2003;111(2):547–553;discussion554–545. doi: 10.1097/01.PRS.0000040466.55214.35 [DOI] [PubMed] [Google Scholar]
- 35.Gurtner GC, Dauskardt RH, Wong VW, et al. Improving cutaneous scar formation by controlling the mechanical environment: large animal and Phase I studies. Ann Surg. 2011;254(2):217–225. doi: 10.1097/SLA.0b013e318220b159 [DOI] [PubMed] [Google Scholar]
- 36.Byrne M, Aly A. The Surgical Suture. Aesthet Surg J. 2019;39(Supplement_2):S67–S72. doi: 10.1093/asj/sjz036 [DOI] [PubMed] [Google Scholar]
- 37.Wang LZ, Ding JP, Yang MY, Chen B. Forty-five cases of chest keloids treated with subcutaneous super-tension-reduction suture combined with postoperative electron-beam irradiation. Dermatol Surg. 2014;40:1378–1384. doi: 10.1097/DSS.0000000000000163 [DOI] [PubMed] [Google Scholar]
- 38.Kazmer DO, Eaves FF 3rd. Force modulating tissue bridges for reduction of tension and scar: finite element and image analysis of preclinical incisional and nonincisional models. Aesthet Surg J. 2018;38:1250–1263. doi: 10.1093/asj/sjy079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Min P, Zhang S, Sinaki DG, et al. Using Zhang’s supertension-relieving suture technique with slowly-absorbable barbed sutures in the management of pathological scars: a multicenter retrospective study. Burns Trauma. 2023;11:tkad026. doi: 10.1093/burnst/tkad026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong VW, Beasley B, Zepeda J, et al. A mechanomodulatory device to minimize incisional scar formation. Adv Wound Care. 2013;2:185–194. doi: 10.1089/wound.2012.0396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wall HC, Halani SH, Mosieri C, et al. Tension reduction with force modulating tissue bridges reduces wounds in breast surgery. Aesthet Surg J. 2023;43:1471–1480. doi: 10.1093/asj/sjad285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qu M, Song N, Chai G, Wu X, Liu W. Pathological niche environment transforms dermal stem cells to keloid stem cells: a hypothesis of keloid formation and development. Med Hypotheses. 2013;81:807–812. doi: 10.1016/j.mehy.2013.08.033 [DOI] [PubMed] [Google Scholar]
- 43.Kim, JY, Willard JJ, Supp DM, et al. Burn scar biomechanics after pressure garment therapy. Plast Reconst Surg. 2015;136(3):572–581. doi: 10.1097/PRS.0000000000001507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engrav LH, Heimbach DM, Rivara FP, et al. 12-year within-wound study of the effectiveness of custom pressure garment therapy. Burns. 2010;36(7):975–983. doi: 10.1016/j.burns.2010.04.014 [DOI] [PubMed] [Google Scholar]
- 45.Shirakami E, Yamakawa S, Hayashida K. Strategies to prevent hypertrophic scar formation: a review of therapeutic interventions based on molecular evidence. Burns Trauma. 2020;8:tkz003. doi: 10.1093/burnst/tkz003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kealey GP, Jensen KL, Laubenthal KN, Lewis RW. Prospective randomized comparison of two types of pressure therapy garments. J Burn Care Rehabil. 1990;11(4):334–336. doi: 10.1097/00004630-199007000-00012 [DOI] [PubMed] [Google Scholar]
- 47.Baur PS, Larson DL, Stacey TR, Barratt GF, Dobrkovsky M. Ultrastructural analysis of pressure-treated human hypertrophic scars. J Trauma. 1976;16(12):958–967. doi: 10.1097/00005373-197612000-00004 [DOI] [PubMed] [Google Scholar]
- 48.Candy LHY, Cecilia L-TWP, Ping ZY. Effect of different pressure magnitudes on hypertrophic scar in a Chinese population. Burns. 2010;36(8):1234–1241. doi: 10.1016/j.burns.2010.05.008 [DOI] [PubMed] [Google Scholar]