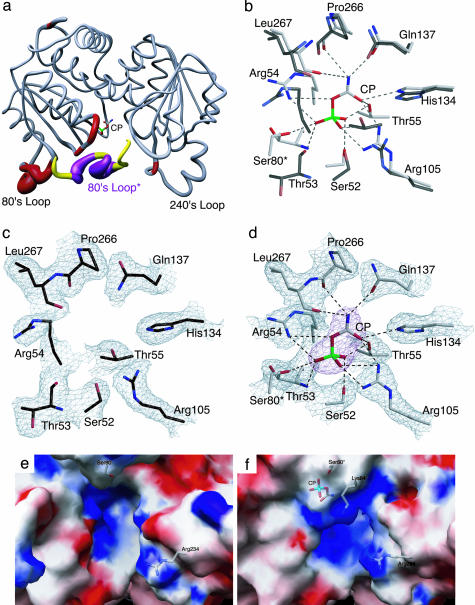
Fig. 1.
Comparison of the x-ray structures of ATCase in the absence and presence of CP. (a) One catalytic chain (gray, red) of ATCase along with the 80's loop of the adjacent catalytic chain (yellow, purple, 80's loop*). The width of the tube is proportional to the rms deviation between the α-carbon positions of the two structures. The CP domain is on the left (with CP shown) and the Asp domain is on the right. (b) Overlay of the CP binding site in the absence and presence of CP. The two structures were first superimposed by using sequoia (37). ATCase in the presence of CP is shown with gray side-chain carbons, whereas the structure without CP is shown with black side-chain carbons. (c) Active site of ATCase in the absence of CP. Orientation is the same as in b. The refined coordinates of the side chains are overlayed on the 2Fo – Fc electron density map shown contoured at 0.8 σ.(d) Active site of ATCase in the presence of CP. Orientation is the same as in b. The refined coordinates of the side chains are overlayed on the 2Fo – Fc electron density map shown contoured at 1.2σ. The CP is overlayed on the Fo – Fc electron density map (magenta) shown contoured at 1.6 σ.(e) Electrostatic potentials mapped onto the surface of ATCase in the absence of CP. (f) Electrostatic potentials mapped onto the surface of ATCase in the presence of CP. In e and f, the positions of Ser-80, Lys-84, Arg-234, and CP are overlaid onto the electrostatic map.