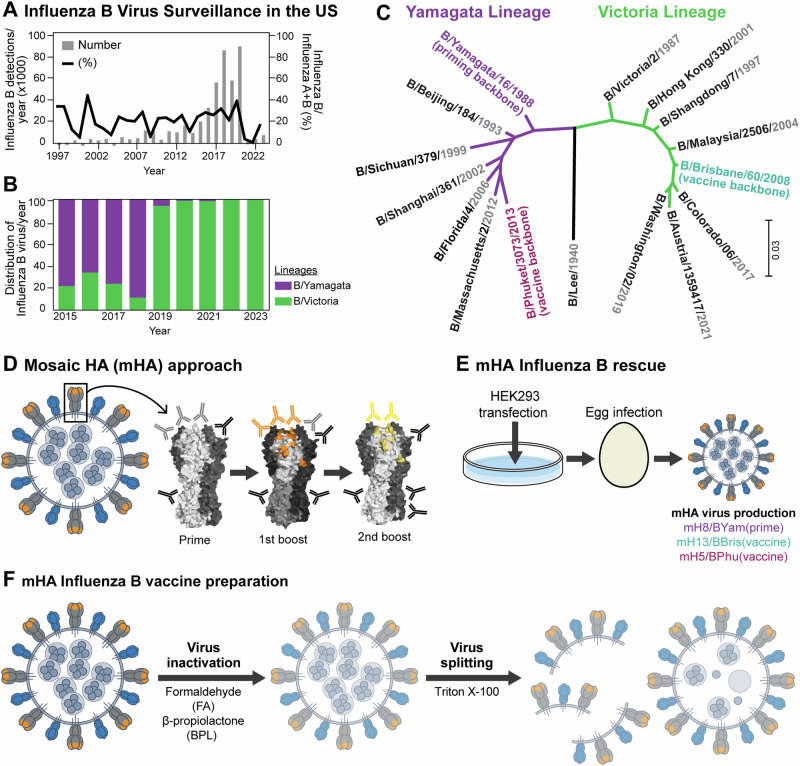
Fig. 1. Influenza B virus surveillance and universal influenza virus vaccine design.
A Surveillance of influenza B viruses in the US from 1997 to 2023 and (B) percentage of influenza B/Yamagata/16/1988-like and B/Victoria/2/1987-like lineage viruses identified from 2015 to 2023 (data obtained from FluNet, (who.int) as of 1st Aug 2023). C Phylogenetic tree of HA sequences of historical annual formulations for IBV vaccine strains from 1999 to 2023 (data obtained from the Global Influenza Programme (who.int) as of 1st Aug 2023 and reported in Supplementary Fig. 113). Influenza B virus HA sequences used in this study as prime vaccination (B/Yamagata/16/1988) and as universal influenza virus vaccines (B/Brisbane/60/2008 and B/Phuket/3073/2013) are highlighted in green and dark red, respectively. The phylogenetic tree was constructed using the maximum likelihood method and Tamura-Nei model and visualized with Mega1163,64. D Mosaic HA (mHA) universal influenza vaccine approach. Sequential vaccination with mHA vaccines, where the major immunodominant epitopes are replaced in each vaccination to refocus the immune response to subdominant head and stalk epitopes of the HA glycoprotein (representation of an HA glycoprotein based on PDB accession no. 4M44). E mHA influenza B virus rescue scheme. B mHA and wildtype (WT) viruses were rescued following the reverse genetics method as previously described17. Thereafter, viruses were propagated in embryonated chicken eggs and harvested in the allantoic fluid. The mosaic viruses were based on B/Yamagata/16/1988 (Yam), B/Brisbane/60/2008 (Bris) and B/Phuket/3073/2013 (Phu) resulting in the mH8/BYam, mH13/BBris and mH5/BPhu viruses, respectively18. F mHA influenza B virus vaccine preparation. Whole inactivated viruses (WIV) were generated by inactivating the harvested viruses with either formaldehyde (FA) or beta-propiolactone (BPL) and purified by sucrose cushion ultracentrifugation. To produce split versions of these vaccines, BPL inactivated and purified virus preparations were treated with Triton X-100 and the remaining detergent was removed using hydrophobic beads in batch mode chromatography19.