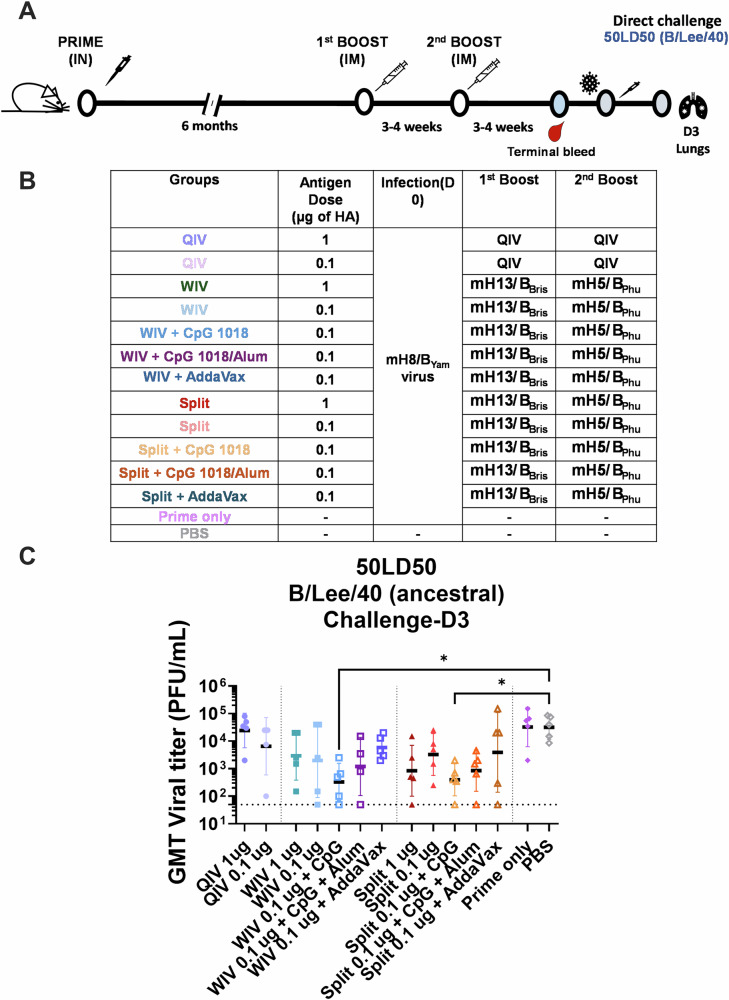
Fig. 5. In vivo cross-protection of mice vaccinated with a low dose of mHA vaccines in a direct virus challenge and serum passive transfer/challenge experiment.
A, B Vaccination regimen and experimental workflow. BALB/c mice were vaccinated in a two-dose vaccination scheme with 1 or 0.1 µg of HA of the different vaccines in a 3–4-week interval after priming with 105 PFU of mH8/BYam virus. WIV or split mHA vaccines were tested without adjuvant or with the addition of CpG 1018 (10 µg), CpG 1018 (10 µg) + Alum (50 µg) or AddaVax (1:1 v:v). A QIV (FluLaval Quadrivalent) vaccinated group and an unvaccinated group (PBS) were included as controls. mH8/BYam virus prime infection was given intranasally in a total volume of 30 µL 6 months prior to the two-dose immunization. C Four weeks after the second dose, mice (n = 5) were challenged with 50× mLD50 dose and viral titers were measured from harvested lung homogenate tissues 3 days post infection. Kruskal–Wallis test corrected using Dunn’s test for multiple comparisons against PBS is shown (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001).