Abstract
Regulated mobile health applications are called digital health applications (“DiGA”) in Germany. To qualify for reimbursement by statutory health insurance companies, DiGA have to prove positive care effects in scientific studies. Since the empirical exploration of DiGA cost-effectiveness remains largely uncharted, this study pioneers the methodology of cohort-based state-transition Markov models to evaluate DiGA for depression. As health states, we define mild, moderate, severe depression, remission and death. Comparing a future scenario where 50% of patients receive supplementary DiGA access with the current standard of care reveals a gain of 0.02 quality-adjusted life years (QALYs) per patient, which comes at additional direct costs of ~1536 EUR per patient over a five-year timeframe. Influencing factors determining DiGA cost-effectiveness are the DiGA cost structure and individual DiGA effectiveness. Under Germany’s existing cost structure, DiGA for depression are yet to demonstrate the ability to generate overall savings in healthcare expenditures.
Subject terms: Health care, Health care economics, Health policy
Introduction
Worldwide 970 million people suffer from mental illness1,2, whereof depression is with around 350 million one major diagnosis1,3–5. The COVID-19 pandemic led to a further increase of depression cases6. Consequently, mental illnesses are the third most common illness causing absence days at work7, with 30.4% of absence days caused by depressive episodes8. At the same time the access to therapy (especially psychotherapy) is limited and waiting times are high - according to estimates by the World Health Organization, only one in four affected people receives adequate treatment4. With increased disease pressure on the one hand and limited treatment capacities on the other, mobile health applications are discussed as new therapeutic option to improve patients’ access to care and generate positive health effects9–14. A meta-analysis of the effect of depression mobile health applications showed a reduction of depressive symptoms of patients using depression mobile health applications15. Germany was the first country worldwide to allow prescription of certified mobile health applications by physicians with cost coverage by statutory health insurances. These apps are called “Digitale Gesundheitsanwendungen” (DiGA) or in English “digital health applications”. Existing research shows that DiGA have significant potential to facilitate patient access to health care and are a valuable complement to existing therapies16–21. To gain prescribing and reimbursement status, DiGA must undergo a comprehensive certification process and provide scientific evidence of effectiveness through clinical trials and compliance with general requirements (e.g., data protection, safety, interoperability)22. In Germany, 42% of all DiGA are developed and approved for mental illnesses, whereof 24% address depression23. These apps offer functionalities such as cognitive-behavioral therapy exercises, mood tracking and psychoeducation in order to provide personalized and accessible mental health care to patients. The effectiveness of DiGA for depression have been demonstrated through clinical studies showing significant improvements in depressive symptoms (e.g., effect size of 1.63 of changes of the Beck Depression Inventory II of one exemplary depression DiGA24). In addition to this, DiGA aim to increase patient engagement and enhance adherence to treatment plans. DiGA are not intended to replace standard therapy, but to support it or offer a bridge to in-person therapy23,25. A study comparing the effectiveness of different internet-based interventions for depression in Germany came to the conclusion that there is a possible superiority of the interventions listed in the DiGA directory compared to other freely available internet interventions26. While the required medical studies mainly focus on the evaluation of the treatment efficacy of DiGA, little is known about the cost-effectiveness of DiGA for the healthcare system21. The development of standard methods and evaluation criteria for the economic benefits of mobile health applications and services in general is challenging due to the great amount of different applications and valuation approaches27–29. As current economic valuation lacks robustness and overarching comparability30, the development of an aggregated value function for assessing the benefits and costs to understand the value of care is still an unaddressed research field27,31–33. Past research developed a pragmatic patient centered framework to assess the economic value of medical evidence of mobile health applications in the United States and the United Kingdom focusing on improvement of quality adjusted life years (QALY)34. A Markov simulation model was previously used to evaluate the cost-effectiveness of mobile health-based integrated care for atrial fibrillation in China35. Another study examined the cost-effectiveness of a DiGA for patients with low back pain in Germany using a state-transition Markov model36. As part of an initiative of the European Commission a “Monitoring and Assessment Framework for the European Innovation Partnership on Active and Healthy Ageing (MAFEIP)” was developed37. This web-based tool allows a comparative assessment of a specific health technology to a standard of care scenario and is based on a Marcov model with three health states in its initial version38.
We aim to develop a methodological approach to financially evaluate the cost-effectiveness of mobile health applications for depression and to provide a calculation and interpretation example for the German DiGA market. Our design criteria were focused on creating a mathematical model that is easily comprehensible and usable by a wide range of researchers and analysts. Thereby we focused on a health insurance perspective and differentiated between different severity levels of depression, namely mild, moderate and severe depression.
Results
Simulation result of the three treatment scenarios
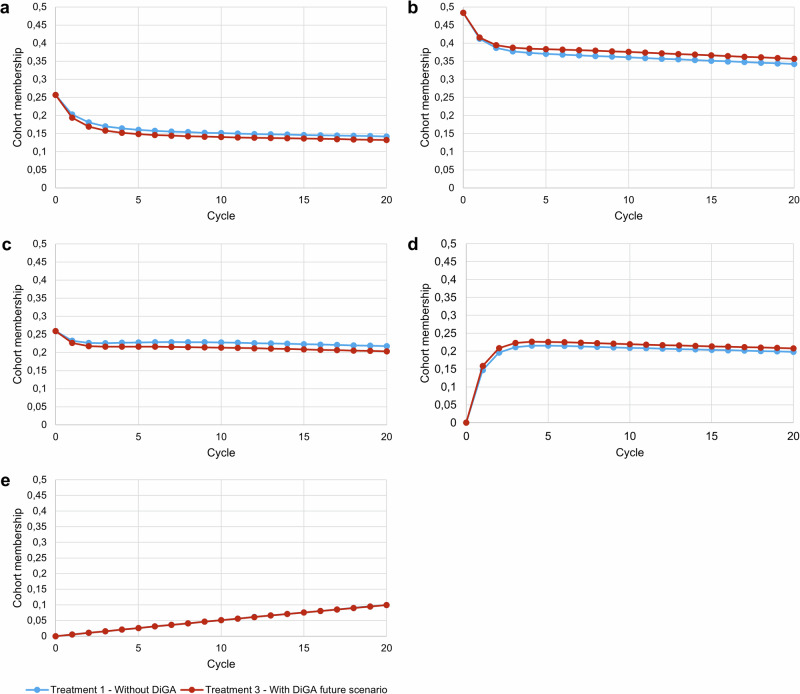
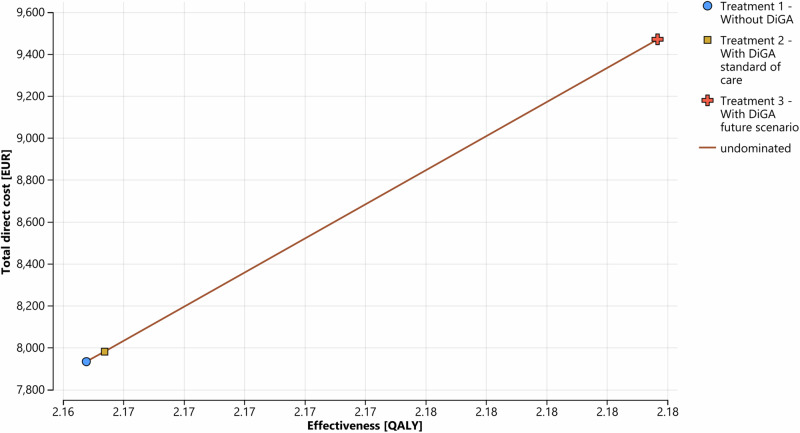
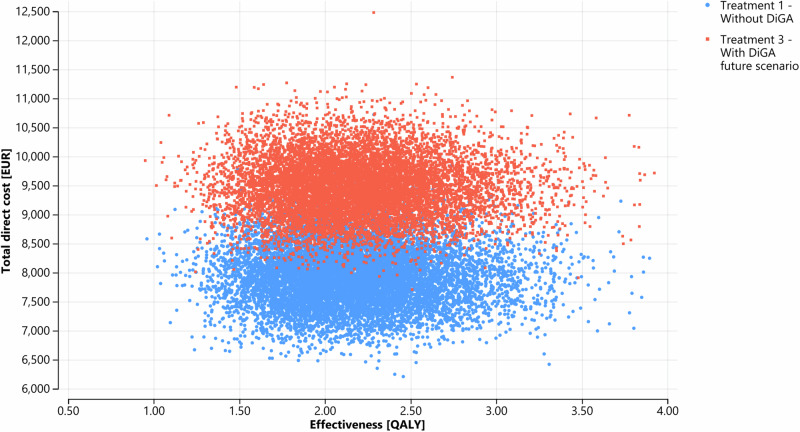
Figure 1a–e shows the development of the patient cohort membership over the simulation horizon for each health state. It can be observed that DiGA treatment effectively delays disease progression as demonstrated by a larger proportion of the cohort remaining in the remission health state and a smaller proportion of the cohort in the severe depression health state. Over the 5 year simulation horizon, the total direct costs of care with DiGA (treatment 2 / 3) and care without DiGA were ~ 39.720 / ~ 47.130 billion EUR and ~ 39.482 billion EUR respectively for the defined patient cohort. Based on our deterministic input data, in total treatment with DiGA is more expensive. The QALY gain was 10.777 / 10.869 million for DiGA treatment. Comparing the future DiGA scenario with the treatment without DiGA, DiGA treatment gained additional QALYs of ~ 0.02 per patient and 94,029 in total for the cohort with an incurred total direct costs of 1536 EUR per patient and ~ 7.648 billion EUR in total over the simulation horizon (In comparison, a study evaluating the cost-effectiveness of enhanced access to psychological therapies found a QALY gain of 0.01439. Another study, which compared cognitive-behavioral therapy with standard care, reported a QALY gain of 0.05340). Table 1 summarizes the results and Fig. 2 shows the cost-effectiveness plane of the three defined treatment strategies. One can observe that neither treatment scenario 2 nor treatment scenario 3 dominates treatment scenario 1. The INMB analysis shows negative values in both DiGA treatment strategies. A negative INMB value indicates that the incremental health effects of an intervention do not outweigh its cost at a given WTP threshold value compared to an alternative intervention41. The ICER analysis shows a higher value than the WTP threshold value of 54,794 EUR for treatment scenario 2 and 3. Consequently, according to the ICER analysis, both DiGA treatment scenarios would not represent a cost-effective treatment alternative compared to treatment scenario 1 without DiGA. Considering a pure economic point of view, treatment scenario 1 would be the option of choice in the base case result given the current cost structure in Germany.
Fig. 1. Markov probability analysis.
Treatment 2 is excluded from the visualization since the value differences to treatment 1 are too marginal to be recognized in the graph. Cycle = 0 represents the starting proportions, cycle = 20 shows the proportions at the end of the simulation horizon. One cycle length is equal to 3 months. a Shows the mild depression health state, b Shows the moderate depression health state, c shows the severe depression health state, d shows the remission health state and e shows the death health state. Blue line = Treatment 1 without DiGA; red line = Treatment 3 with DiGA future scenario. The Markov probability analysis graphs show the proportions of the cohort in treatment 1 and treatment 3 belonging to the defined health states over the simulation horizon.
Table 1.
Simulation result using deterministic input variables (simulation horizon of 5 years)
| Treatment scenario | Cumulated total direct costs base case [in EUR] | Cumulated effectiveness base case [in QALY] | Incremental net monetary benefit (INMB), comparator: Treatment 1 [in EUR] | Incremental cost effectiveness ratio (ICER), comparator: Treatment 1 [EUR/QALY] | Ranking treatment scenarios | ||
|---|---|---|---|---|---|---|---|
| Per patient | Per cohort | Per patient | Per cohort | ||||
| Treatment 1: Without DiGA scenario | 7,933 | ~39,482 million | 2.16478 | 10,774,482 | 1 | ||
| Treatment 2: With DiGA scenario standard of care | 7,980 | ~39,720 million | 2.16538 | 10,777,473 | -14.84 | 79,466 | 2 |
| Treatment 3: With DiGA future scenario | 9,469 | ~47,130 million | 2.18367 | 10,868,511 | -501.43 | 81,335 | 3 |
Fig. 2. Cost-effectiveness plane.
The graph shows the effectiveness and total direct costs of each treatment scenario over the simulation horizon and per patient. Blue dot = Treatment 1 without DiGA; yellow square = Treatment 2 with DiGA standard of care; red cross = Treatment 3 with DiGA future scenario.
Sensitivity analysis
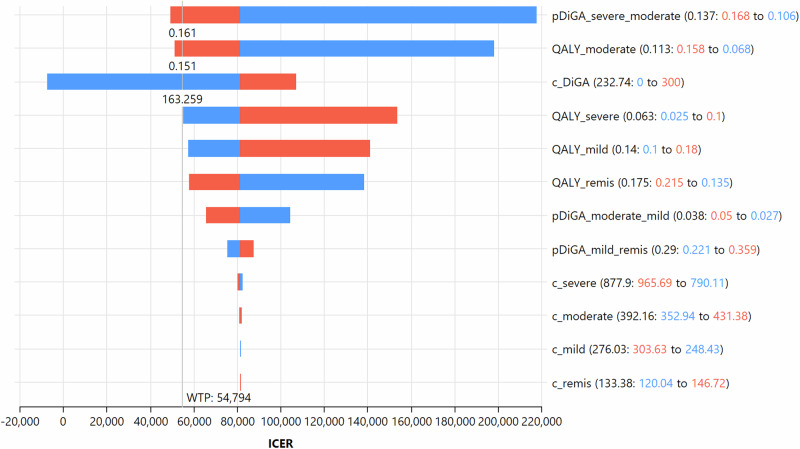
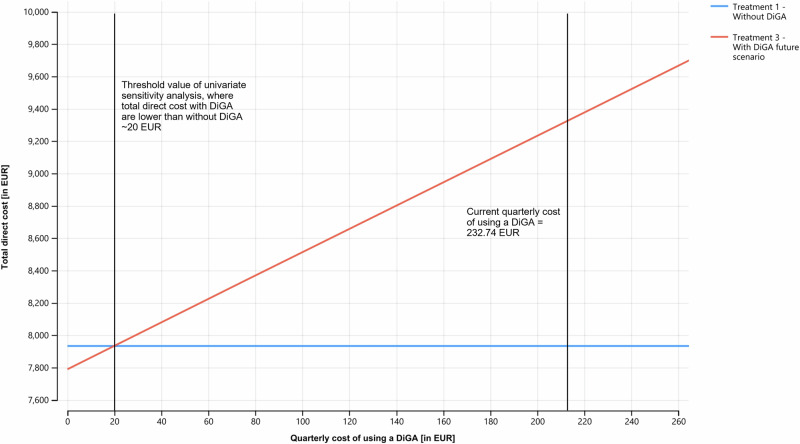
Univariate sensitivity analysis (also called one way sensitivity analysis) was performed to assess the robustness of the base case results (see Fig. 3). We excluded treatment 2 from the sensitivity analysis for better readability because it only differs from treatment 3 in terms of the proportion of the cohort using a DiGA, resulting in identical interpretation of the results for both treatment strategies. The analysis showed that the model results were most sensitive to the following three input variables: the transition probability moving from the severe depression health state to the moderate one, the utility value for the moderate depression health state and the quarterly cost for using a DiGA. We performed an extended one-way sensitivity analysis for the quarterly cost of using a DiGA (see Fig. 4). Once the quarterly price for getting a DiGA would reach a threshold value below 20.34 EUR per quarter, both DiGA treatment scenarios would represent a cost-effective and dominant scenario.
Fig. 3. Tornado diagram of one-way sensitivity analysis assessing the effect of selected parameters on the ICER.
ICER = Incremental cost effectiveness ratio, WTP = willingness-to-pay ratio referred to quality-adjusted life years; the horizontal bars represent the range of ICER due to changes in the model’s input parameters for one average patient in the cohort. Codification in the legend according to the following scheme: Input variable name (base case value: upper/lower range to lower/upper range), red = upper range of the input variable variation; blue = lower range of the input variable variation. p_DiGA_severe_moderate = transition probability to move from severe to moderate depression health state with use of DiGA; QALY_moderate = quarterly quality adjusted life years of an average patient in moderate depression health state; c_DiGA = quarterly cost of using a DiGA; QALY_severe = quarterly quality adjusted life years of an average patient in severe depression health state; QALY_mild = quarterly quality adjusted life years of an average patient in mild depression health state; QALY_remis = quarterly quality adjusted life years of an average patient in remission health state; pDiGA_moderate_mild = transition probability to move from moderate to mild depression health state with use of DiGA; pDiGA_mild_remis = transition probability to move from mild depression to remission health state with use of DiGA; c_severe = quarterly total direct costs of an average patient in severe depression health state; c_moderate = quarterly total direct costs of an average patient in moderate depression health state; c_mild = quarterly total direct costs of an average patient in mild depression health state; c_remis = quarterly total direct costs of an average patient in remission health state.
Fig. 4. Extended univariate sensitivity analysis of input variable quarterly cost of using a DiGA.
The graph shows the comparison of total direct costs of treatment scenario 1 and treatment scenario 3 depending on the quarterly cost of using a DiGA. The analysis shows that once the quarterly cost of using a DiGA fall below the threshold of ~20 EUR, the total direct costs of scenario 3 are lower compared to scenario 1. Blue line = Treatment 1 without DiGA; red line = Treatment 3 with DiGA future scenario.
Probabilistic sensitivity analysis (PSA) helped to determine input parameter uncertainty. Figure 5 shows the result of the PSA based on 10,000 simulation runs. DiGA treatment gained average QALYs of 2.19 (95% CI; 2.18–2.20 QALY) with mean total direct costs of 9476 EUR (95% CI; 9466–9486 EUR) per patient in the cohort; compared to care without DiGA with average QALYs of 2.17 (95% CI; 2.16–2.18 QALY) with mean total direct costs of ~ 7,936 EUR (95% CI; 7926–7946 EUR) per patient in the cohort. Of 10,000 iterations, the probability of DiGA treatment being effective in QALY gains with cost savings was 36.15% in our exemplary PSA.
Fig. 5. Probabilistic sensitivity analysis (PSA).
Each data point marks the result of one of the 10,000 simulation runs, where input parameters were randomly drawn from defined distributions according to Table 2 to assess parameter uncertainty. DiGA treatment gained average QALYs of 2.19 (95% CI; 2.18–2.20 QALY) with mean total direct costs of ~ 9476 EUR (95% CI; 9466–9486 EUR) per patient in the cohort; compared to the treatment without DiGA with average QALYs of 2.17 (95% CI; 2.16–2.18 QALY) with mean total direct costs of ~7936 EUR (95% CI; 7926–7946 EUR) per patient in the cohort. Blue dots = Treatment 1 without DiGA; red dots = Treatment 3 with DiGA future scenario.
Discussion
Our cohort-based state-transition Markov model (CMM) revealed that DiGA for depression, when used on an add-on basis, with a probability of 63.85% do not directly save costs but can improve patient care and enhance public health outcomes. Inaccessible therapy for depression is a challenge, with two-thirds of depressed individuals not seeking professional help within a year due to general lack of availability and regional disparities in psychotherapy availability42. In contrast, DiGA offer nationwide accessibility and can potentially bridge waiting times for psychotherapy and thus help that symptoms do not deteriorate. Hence, DiGA can improve the care for underserved populations or subgroups and reduce overall morbidity43.
Based on our CMM, we concluded that DiGA for the depression on the one hand can help to improve the quality of life of people suffering from depression, but on the other hand are associated with noticeable extra costs and hence currently represent no dominant health strategy. These findings are in line with past research. Kolovos, S. et al.44 used multilevel regression analysis to assess the cost-effectiveness of internet interventions for depression (intervention was not a DiGA) concluded that they are more costly and not considered as cost-effective in comparison to the defined control group.
We further hypothesize that the cost dominance of DiGA treatment strategies is dependent on the respective indication group due to different cost structures and treatment options. Lewkowicz et al.36 analyzed the cost-effectiveness of a DiGA for patients with low back pain. Their base case analysis revealed additional costs of 121.59 EUR with QALY gains of 0.0221. Since they used a different time horizon in their base case analysis, we compared their model results of a 5-year time horizon scenario analysis to our results. In this scenario analysis, DiGA treatment for low back pain seemed to be a dominant treatment strategy since their model revealed cost savings of 381.80 EUR with QALY gains of 0.0534.
For our cost-effectiveness analysis we used a depression specific WTP threshold for one QALY. Germany’s health care system offers some special characteristics in this context. By law, there is an obligation for all Germans to have a health insurance and for patients with statutory health insurances, a large proportion of all healthcare services is covered by the insurances. For this reason, past studies have shown that citizens in Germany have difficulties estimating WTP threshold values for hypothetical scenarios since they are used to health insurances covering all costs without them having any transparency regarding costs45. Generalized, disease-independent WTP thresholds are even seen as non-compliant with the Social Security Law Book V in Germany46,47.
Next, our results indicate that the quarterly price for using a DiGA are one lever to build a dominant DiGA treatment scenario. While on the one hand current DiGA prices might be justifiable to cover development costs and to foster innovation in a relatively new field of medical innovation, on the other hand statutory health insurances criticize the additional cost constraint resulting from DiGA prices48–50. Following the cost development dynamic of pharmaceuticals over their life cycle, we expect DiGA prices to fall in the long run. Nevertheless, there is optimization potential in aligning the interests of both DiGA providers and statutory health insurances to achieve higher prescription and usage rates. Our CMM can be used by both DiGA providers and statutory health insurances to determine their individual WTP threshold value for a DiGA treating depression. Concerns raised by health insurances align with findings from a study analyzing DiGA application reviews in Germany, indicating that end users also question current pricing mechanisms21. Gräfe, V. et al.51 examined the economic potential of one specific DiGA for depression using administrative data of a statutory health insurance in Germany. The authors found that the difference of the total cost decrease of the intervention group compared to the control group was significant, although the difference of costs in the single cost categories was not significant. Additional scenario analysis revealed that if the DiGA usage cost exceeded 34 euro per patient, there would no longer be a significant difference of total cost of both treatment groups. Comparing this to our threshold value of ~20 EUR per patient and quarter, we are in a similar range. Any observed difference could be attributed to variations in the cost baseline, as we included outpatient costs in our CMM.
The difference of results of the QALY effect of treatment 1 and 2 is marginal. This effect can be explained by the current DiGA usage numbers: If we relate the approximate current usage figures for depression DiGA in Germany to the total number of patients suffering from depression and extrapolating it to our simulation horizon, the number accounts to 2% over the 5 year simulation horizon (used as input factor for treatment 2). Thus, we conclude that current DiGA usage numbers have a marginal effect on the German health care system. The Association of Statutory Health Insurance Funds in Germany (“GKV Spitzenverband”) reports a steady growth rate of total DiGA usage (200% from October 2021 to September 2022 and 68% from October 2022 to September 202352). Combined with an assumed increasing and improved digital affinity among the German population, higher usage numbers can be expected in the future – which we accounted for in our treatment scenario 3. At the same time the association of providers of DiGA demands to simplify the access to DiGA for patients. They report that on average patients wait 13 days to receive a so-called DiGA activation code resulting in a delayed start of the therapy and potential further deterioration of symptoms. In addition, DiGA providers underscore the therapeutic autonomy of physicians, since they report of instances where statutory health insurances have recommended less expensive mobile health applications (instead of the described DiGA) to their policyholders that lack the approval form the Federal Institute for Drugs and Medical Devices in Germany53.
We further like to emphasize that current data availability and comparability is a major issue that needs to be addressed. We invested a considerable amount of time in researching and synthetizing suitable, robust, and comparable input data form various data sources. We hence argue that while it is essential to further invest in systematic evaluation procedures and methodologies in healthcare28, the provision of interoperable real-world data for researchers is crucial. Real-world data helps to continuously learn and evaluate the benefits, costs, chances and challenges of DiGA in everyday care to provide a sustainable and efficient resource allocation as well as strategic improvement of healthcare17.
Some limitations need to be considered when interpreting our results. The overall data quality of our exemplary base case scenario is one limitation. First, our input data only refers to the German healthcare system and hence lacks in international comparability. Secondly, as previously noted, we had to aggregate data from various sources with different publication years, and we opted not to apply a time correction for the sake of clarity. Thirdly, regarding the transition probabilities, to the best of our knowledge, there is only one available data source that we could utilize to derive the underlying transition probabilities for treatment 1, specifically considering various depression severity levels (“without DiGA”). Concerning the transition probabilities for treatment 2 and 3, we used several DiGA studies which all have a slightly different study set-up, e.g., the control groups are not analogously defined or the availably of guided versus unguided therapy was differently handled. For the costs, we only included direct costs, but one could argue that indirect costs, such as productivity losses through depression, and other qualitative factors, like technical features, perceived benefits from patients or physicians or process efficiency gains etc. are not considered. Furthermore, the limitations of the input data also caused a necessary simplification in the development of our CMM: We have not considered the comorbidity of depression, although past research has shown that depression is always comorbid with other mental disorders like, for example, anxiety54. Beyond that, for reasons of simplicity, we used discrete instead of continuous modeling of health states and transition probabilities and did not account for individual patient histories. The risk of recurrence in depression depends on the number of previous depressive episodes55 such that the inclusion of patients’ histories would probably better represent the real course of the disease. This comes with the practical downsides of greater model complexity and higher input data requirements.
Hence, further research could validate our developed CMM with real-world data for transition probabilities, costs, and utilities. If real world data is available, it would be of great value to also incorporate indirect cost factors due to the significant impact of productivity losses of depression patients. We expect that the cost-effectiveness of DiGA would improve if the total costs per health state were increased by adding indirect costs. If the data availability hurdle is solved in the future, an individual-based state-transition model could be built and compared to our CMM model. Furthermore, our developed model can be applied and compared to different countries. Further research could also invest in building models to assess the cost-effectiveness of mobile health applications of further diseases.
We applied a successful proof-of-concept for the application of a CMM model to simulate the cost-effectiveness of depression DiGA in Germany. Our developed CMM can be used as standard framework for decision makers to assess the cost-effectiveness of mobile health applications. Our results show that DiGA for depression in Germany are an innovative opportunity to increase the overall QALYs of a population and hence can contribute to the improvement and access of health care, but given the current cost structure in Germany, DiGA cannot yet contribute to save total health care costs.
Methods
Model selection and structure
Decision trees, cohort-based state-transition Markov models (CMM) or individual-based state-transition models are commonly used to develop cost-effectiveness models for depression treatments56. We used a CMM to evaluate the cost-effectiveness of DiGA for depression compared to the current standard of care without DiGA. Decision criteria to use a CMM were their transparency, flexibility, efficiency, ease of debugging, intuitiveness and suitability for depression57. Compared to decision trees, Markov models can better simulate different disease courses and hence have better clinical validity for depression56. While individual-based state-transition models could account for single patient characteristics and histories, we opted against them due to greater model complexity and the lack of suitable input data (CMMs require lower input data requirements)56. We applied the best practice guidelines of the ISPOR-SMDM Modeling Good Research Practices57,58 for the development of our model (see Supplementary Table 1). In addition, the CHEERS 2022 checklist for health economic evaluations was applied and can be found in Supplementary Table 259,60.
CMMs are used to illustrate the progression of disease through a sequence of changes between defined health states. The model is a discrete-time model dividing the time horizon into cycles of equal length. Within CMMs, a cohort of patients move from one state to another or remain in the same state within one cycle. The transitions between the defined health states are defined by conditional, so-called transition probabilities which depend on the current health condition only. CMMs are therefore called memoryless (“Markovian property”), meaning that a future health state only depends on the current health state and not the sequence of previous ones57,61. Consequently, CMMs do not account for patient histories or the time a single patient spends in one health state56. The transition between discrete health states persists until patients reach an irreversible state like “death” or until the specified time horizon is reached. Costs and utilities are assigned to each defined health state. The total anticipated costs and utilities are calculated across the model’s overall time horizon by summarizing the duration spent in each health state and multiplying it by the corresponding costs and utility weights55.
To assess the cost-effectiveness of depression mobile health apps, we defined three treatment scenarios. These scenarios help gauge the value of the digital health solution by comparing its incremental advantages to the existing standard of care31. The first one describes the standard of care before the introduction of mobile health applications (in Germany DiGA), hereafter referred to “Treatment 1: Without DiGA”. The second one describes the current standard of care, where patients have additionally access to mobile health applications/DiGA and current usage rates are used as basis (“Treatment 2: With DiGA standard of care”). The third one describes a hypothetical future scenario with mobile health applications/DiGA, where we expect the DiGA usage rate to increase (“Treatment 3: With DiGA future scenario”). We assume that 50% of the cohort use a DiGA. Previous studies concluded an average treatment rate of affective disorders in Germany of ~50%, which we accordingly set as future potential DiGA treatment threshold in our study62,63.
The state transition diagram of the CMM is illustrated in Fig. 6. We differentiated between different severity levels for depression (mild, moderate, severe depression) since we need this level of detail to model the shown symptom improvements through mobile health applications. The “remission” health state refers to a period during the course of depression where the symptoms are reduced or disappear completely (recovery). We built our model in the software TreeAge Pro, version 2023. Supplementary Fig. 1a-c shows the implemented model structure in TreeAge Pro.
Fig. 6. State transition diagram of the CMM model.
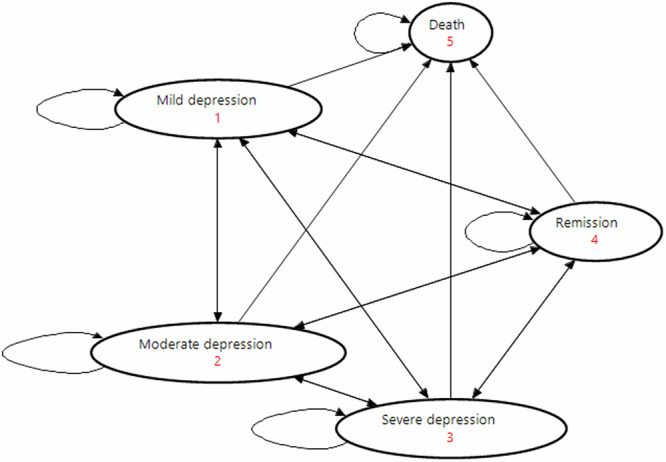
The arrows show the possible transition paths of the patient cohort. The red numbers show the numbering of the health states. This graph shows the five modeled health states in the CMM model (mild depression, moderate depression, severe depression, remission, death).
Base case input data and sensitivity analysis
The base case data contains deterministic input data for the three treatment scenarios. In our example, these values are determined based on the German healthcare system. They serve as initial reference points to initiate the assessment of the cost-effectiveness of DiGA. Our model requires input data around transition probabilities, costs and utilities per health state, proportions about the starting cohort and specific CMM parameters.
To conduct cost-effectiveness analysis, we used both the incremental net monetary benefit (INMB) as well as the incremental cost-effectiveness ratio (ICER) approach according to Eq. (1) and Eq. (2). Both measures need a willingness-to-pay (WTP) threshold value as well as incremental cost and effectiveness values as input data. Incremental cost and effectiveness are calculated by comparing the DiGA treatment scenarios (Treatment 2 or 3) with a treatment without DiGA (Treatment 1).
| 1 |
| 2 |
A treatment scenario is considered cost-effective in case ICER < WTP or INMB > 064,65. The WTP threshold value describes the amount the patient cohort is willing to pay on average for one QALY. The specific WTP threshold can vary widely within one country depending on for example the current health status, income level and level of education45. We base our analysis on a study of Ulbrich & Kröger66 that analyzed WTP threshold values for major depressive disorders in different scenarios. Within the patient sample, the mean WTP thresholds vary between 15,778 EUR and 54,794 EUR (median 13,000 EUR to 15,000 EUR). In consultation with the authors of this study, the upper value was found most appropriate as WTP threshold value for our use case, which corresponds to 1.12 times the 2023 gross domestic product in Germany per inhabitant67. To assess uncertainties in the deterministic input data, it is recommended to conduct univariate and probabilistic sensitivity analysis (PSA)68–70. For the PSA, we assigned probability distributions to input parameters based on existing research69,71. Usually, beta distributions are used to model the distribution for probabilities and utilities, whereas gamma distributions are used for costs72. We used beta distributions to model uncertainty in the transition probabilities and utility values since they are naturally bounded between 0 and 1 and are highly versatile73. Gamma distributions are used for costs because the distribution is defined for positive values only and can represent a wide range of positively skewed data72. The mean of the distributions represents the deterministic base case value, whereas the standard deviation is either derived from the original studies that were used to determine the base case value74 or where limited data was available we used a +/-10% range of the base case value75. Additionally, clinical practitioners validated the data. We tested several numbers of simulation runs, i.e., 1000; 10,000; and 100,000 runs by varying the defined input variables simultaneously with random draws from each defined distribution. For the univariate sensitivity analysis, we calculated a range for selected input variables by subtracting or adding one standard deviation from the base case value to examine the impact of selected input parameters on the ICER. Since the quarterly price for using a DiGA is a widely discussed parameter, we explicitly modeled a wider spread of this input parameter65.
Exemplary input parameters
Table 2 summarizes the input parameters with exemplary numbers for the German health care system. The time horizon for the CMM was set at 5 years with each simulation cycle lasting 3 months since DiGA are usually prescribed for this period50. As cohort size we chose 4,977 million which represents the prevalence of depression in the German society76,77. For the probabilities of transitioning under “Treatment 1: Without DiGA scenario”, key insights were drawn from a study analyzing the state of care of patients with depression78. This study relies on data sourced from diagnosis and billing records within the German statutory health insurance system. As a limiting factor the authors calculated yearly transition probabilities, which we used for our CMM on a quarterly basis. Furthermore, the authors reported “no diagnosis” transitions, which we interpreted as our remission health state. The transition probabilities for treatment 2 and 3 are derived from the German DiGA studies employing a three-step approach. First, we analyzed the publicly available medical studies of permanently listed depression DiGA listed in the DiGA directory as of October 17, 2023. Our analysis centered on primary outcome parameters that are consistent across all selected studies, the Beck Depression Inventory (BDI and BDI-II) as well as the Patient Health Questionnaire-9 (PHQ-9). We selected the intention-to-treat data with the aim to provide a realistic insight into DiGA effectiveness while at the same time considering therapy adherence to ascertain the actual utilization of DiGA. Second, we translated the BDI/BDI-II and PHQ-9 improvement scores into the ICD-10-CM depression classification to facilitate the mapping of the depression symptom improvements to our defined health states using the assumption of normative distribution data of BDI-II and PHQ-979–84. Third, we calculated the improvement of the transition probabilities, e.g. a specific percentage improvement through DiGA treatment translates in an increase of the transition probability from the current health state to the “next better” health state and a respective decrease of the current health state (e.g., 45% improvement of moderate depression translates in an increase of the transition probability from moderate to mild and a decrease of the transition probability from moderate to staying in moderate). Supplementary Table 3 summarizes the included DiGA studies. To assign cost values to the defined health states, we focused on direct cost factors only, i.e. costs for pharmaceuticals, outpatient, and inpatient costs. Costs for psychotherapy, general practitioner (GP) visits and psychiatrist visits make up the outpatient costs. Indirect costs, that are not directly related to the intervention such as productivity losses or rehabilitation costs, are not considered to narrow the scope of the analysis. To determine exemplary data for the German population depending on depression severity, we followed a bottom-up approach and aggregated the inputs from the data sources listed in Table 2. We conducted a double validation process of our exemplary cost data—on the one hand with experts from the specific field and on the other hand by means of a top-down validation with published aggregated data of the Federal Statistical Office of Germany85. To measure the utility of the defined health states we used health related quality adjusted life years (QALYs) operationalized by the frequently-used EQ-5D instrument86. A QALY is a measure in health economics that combines the quantity (time in years) and quality of life (utility value). All cost and utility values per health state were discounted87 and we applied half-cycle correction. Since our study was based on publicly available data, an Ethics Committee Approval was not required.
Table 2.
Model inputs of probabilities, costs and utilities
| Variable name | Description | Base-case value | Distribution (standard deviation) | Reference |
|---|---|---|---|---|
| CMM parameters | ||||
| _stage | Number of cycles simulated | 20 | -- | Predefined value |
| cycle_length | Duration of one cycle | 3 months | -- | Predefined value |
| discount_rate | Discount rate for cost values | 0.004 | -- | Deutsche Bundesbank88 |
| discount_rate_QALY | Discount rate for QALYs | 0.004 | -- | Deutsche Bundesbank88 |
| p_getDiGA | Proportion of total patient cohort using a DiGA |
Treatment 2: 0.0154 Treatment 3: 0.5 |
-- |
Treatment 2: GKV Spitzenverband52 |
| Cohort size | Total cohort size represents amount of people in Germany with self-reported diagnosis of depression | 4,977,172 | -- | Grobe et al.76, Statistisches Bundesamt77 |
| Proportion of starting cohort | ||||
| p_mild | Proportion of cohort starting in the mild depression health state | 0.257 | -- | Stahmeyer et al.78 |
| p_moderate | Proportion of cohort starting in the moderate depression health state | 0.484 | -- | Stahmeyer et al.78 |
| p_severe | Proportion of cohort starting in the severe depression health state | 0.259 | -- | Stahmeyer et al.78 |
| p_remis | Proportion of cohort starting in the remission health state | 0.000 | -- | Predefined value |
| p_death | Proportion of cohort starting dead | 0.000 | -- | Predefined value |
| Transition probabilities | ||||
| pwo_mild_mild |
Transition probabilities of treatment 1: Without DiGA scenario (pwo) Codification according to the following scheme pwo_a_b, where a indicates the health state of the current cycle and b the health state in the next cycle |
0.717 | Calculated* | Stahmeyer et al.78 |
| pwo_mild_moderate | 0.055 | Beta (0.006) | Stahmeyer et al.78 | |
| pwo_mild_severe | 0.022 | Beta (0.002) | Stahmeyer et al.78 | |
| pwo_mild_remis | 0.200 | Beta (0.020) | Stahmeyer et al.78 | |
| pwo_mild_death | 0.005 | Beta (0.001) | Statistisches Bundesamt89,90 | |
| pwo_moderate_moderate | 0.774 | Calculated* | Stahmeyer et al.78 | |
| pwo_moderate_mild | 0.027 | Beta (0.003) | Stahmeyer et al.78 | |
| pwo_moderate_severe | 0.047 | Beta (0.005) | Stahmeyer et al.78 | |
| pwo_moderate_remis | 0.147 | Beta (0.015) | Stahmeyer et al.78 | |
| pwo_moderate_death | 0.005 | Beta (0.001) | Statistisches Bundesamt89,90 | |
| pwo_severe_severe | 0.787 | Calculated* | Stahmeyer et al.78 | |
| pwo_severe_mild | 0.021 | Beta (0.002) | Stahmeyer et al.78 | |
| pwo_severe_moderate | 0.091 | Beta (0.009) | Stahmeyer et al.78 | |
| pwo_severe_remis | 0.094 | Beta (0.009) | Stahmeyer et al.78 | |
| pwo_severe_death | 0.006 | Beta (0.001) | Statistisches Bundesamt89,90 | |
| pwo_remis_remis | 0.495 | Calculated* | Stahmeyer et al.78 | |
| pwo_remis_mild | 0.129 | Beta (0.013) | Stahmeyer et al.78 | |
| pwo_remis_moderate | 0.242 | Beta (0.024) | Stahmeyer et al.78 | |
| pwo_remis_severe | 0.130 | Beta (0.013) | Stahmeyer et al.78 | |
| pwo_remis_death | 0.005 | Beta (0.001) | Statistisches Bundesamt89,90 | |
| pwo_death_death | 1.000 | -- | ||
| pDiGA_mild_mild |
Transition probabilities of treatment 2 and 3: With DiGA scenario standard of care/future (pDiGA) Codification according to the following scheme pDiGA_a_b, where a indicates the health state of the current cycle and b the health state in the next cycle |
0.627 | Calculated* |
Stahmeyer et al.78; Klein et al.11; Meyer et al.91; Moritz et al.92,93; Krämer et al.24; Beiwinkel et al.94; Baumeister & Moritz95 See Supplementary Table 3 for overview of included DiGA studies Distribution: Kligerman et al.75 ; standard deviations of included DiGA studies |
| pDiGA_mild_moderate | 0.055 | Beta (0.006) | ||
| pDiGA_mild_severe | 0.022 | Beta (0.002) | ||
| pDiGA_mild_remis | 0.290 | Beta (0.069) | ||
| pDiGA_mild_death | 0.005 | Beta (0.001) | ||
| pDiGA_moderate_moderate | 0.763 | Calculated* | ||
| pDiGA_moderate_mild | 0.038 | Beta (0.012) | ||
| pDiGA_moderate_severe | 0.047 | Beta (0.005) | ||
| pDiGA_moderate_remis | 0.147 | Beta (0.015) | ||
| pDiGA_moderate_death | 0.005 | Beta (0.001) | ||
| pDiGA_severe_severe | 0.741 | Calculated* | ||
| pDiGA_severe_mild | 0.021 | Beta (0.002) | ||
| pDiGA_severe_moderate | 0.137 | Beta (0.031) | ||
| pDiGA_severe_remis | 0.094 | Beta (0.009) | ||
| pDiGA_severe_death | 0.006 | Beta (0.001) | ||
| Costs [in EUR] | ||||
| c_mild | Quarterly direct costs of an average patient in mild depression health state | 276.03 | Gamma (27.60) |
Pharmaceuticals: Bundesinstitut für Arzneimittel und Medizinprodukte96; Wissenschaftliches Institut der AOK97; IGES Institut98; Stahmeyer et al.78 Outpatient: Kassenärztliche Bundesvereinigung99; Stahmeyer et al.78; Expert judgement Inpatient: GKV Spitzenverband100; Statista101; Osterloh102 Overall: Bundesärztekammer et al.82; see Supplementary Table 3 for overview of included DiGA studies Distribution: Kligerman et al.75 |
| c_moderate | Quarterly direct costs of an average patient in moderate depression health state | 392.16 | Gamma (39.22) | |
| c_severe | Quarterly direct costs of an average patient in severe depression health state | 877.90 | Gamma (87.79) | |
| c_remis | Quarterly direct costs of an average patient in remission health state | 133.38 | Gamma (13.34) | |
| c_DiGA | Quarterly cost of an average patient using a depression DiGA | 232.74 | Gamma (20.76) | |
| c_health state_disc (_stage) | Discounted cost values per health state depending on current cycle | |||
| Utilities | ||||
| QALY_mild | Quarterly quality adjusted life years (QALY) of an average patient in mild depression health state | 0.140 | Beta (0.040) | Mohiuddin & Payne103 |
| QALY_moderate | Quarterly quality adjusted life years (QALY) of an average patient in moderate depression health state | 0.113 | Beta (0.045) | Mohiuddin & Payne103 |
| QALY_severe | Quarterly quality adjusted life years (QALY) of an average patient in severe depression health state | 0.063 | Beta (0.038) | Mohiuddin & Payne103 |
| QALY_remis | Quarterly quality adjusted life years (QALY) of an average patient in remission health state | 0.175 | Beta (0.040) |
Kolovos et al.104 Distribution: Revicki & Wood105 |
| QALY_health state_disc (_stage) | Discounted quality adjusted life years (QALY) per health state depending on current cycle | |||
*Transition probability in probabilistic sensitivity analysis is calculated according to the following formula: 1-sum of all remaining transition probabilities of respective health state, e.g. pwo_mild_mild=1-pwo_mild_moderate-pwo_mild_severe-pwo_mild_remis-pwo_mild_death.
Supplementary information
Acknowledgements
B.F. received a doctoral scholarship granted by Ev. Studienwerk Villigst for gifted students. Apart from that, this study received no funding.
Author contributions
B.F. and L.F. conceived the study. B.F., L.F. and S.M. designed the study. B.F. collected data, programmed the model and performed analyses. B.F. and L.F. drafted the manuscript. L.F., M.U. and S.M. provided critical review. M.U. conducted the English proof reading. B.F., L.F., C.P. and S.M. revised the manuscript. L.F. coordinated the project. All authors have read and approved the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
The code that supports the findings of this study is available from the corresponding author upon reasonable request.
Competing interests
All authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41746-024-01324-0.
References
- 1.Vigo, D., Thornicroft, G. & Atun, R. Estimating the true global burden of mental illness. Lancet Psychiatry3, 171–178 (2016). [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Regional Office for Europe. WHO European Framework for Action on Mental Health 2021–2025. https://www.who.int/europe/health-topics/mental-health#tab=tab_1 (2022).
- 3.Robert Koch-Institut. Gesundheit in Deutschland. Gesundheitsberichterstattung des Bundes.https://www.destatis.de/DE/Themen/Gesellschaft- (2015).
- 4.Bundesministerium für Bildung und Forschung. Depression: Schatten auf der Seele.https://www.gesundheitsforschung-bmbf.de/de/depression-schatten-auf-der-seele-5949.php (2023).
- 5.Porst, M. et al. The burden of disease in Germany at the national and regional level. Dtsch Arztebl Int119, 785–792 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabe-Menssen, C., Hübner, L. & Maaß, E. Report Psychotherapie 2023. Sonderausgabe psychische Gesundheit in der COVID-19-Pandemie.https://www.dptv.de/im-fokus/wissenschaft-und-forschung/report-psychotherapie/ (2023).
- 7.DAK Gesundheit. Psychreport 2023. Entwicklungen der psychischen Erankungen im Job: 2012-2022.https://www.dak.de/dak/unternehmen/reporte-forschung/psychreport-2023_32618#rtf-anchor-downloads-psychreport-2023-ergebnis-prasentation (2023).
- 8.Grobe, T. G., Braun, A. & Starke, P. BARMER Gesundheitsreport 2023. Risikofaktoren für psychische Erkrankungen, Schriftenreihe zur Gesundheitsanalyse - Band 41. https://www.bifg.de/publikationen/reporte/gesundheitsreport-2023 (2023).
- 9.Barak, A., Hen, L., Boniel-Nissim, M. & Shapira, N. A comprehensive review and a meta-analysis of the effectiveness of internet-based psychotherapeutic interventions. J. Technol. Hum. Serv.26, 109–160 (2008). [Google Scholar]
- 10.Karyotaki, E. et al. Do guided internet-based interventions result in clinically relevant changes for patients with depression? An individual participant data meta-analysis. Clin. Psychol. Rev.63, 80–92 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Klein, J. P. et al. Effects of a psychological internet intervention in the treatment of mild to moderate depressive symptoms: Results of the EVIDENT study, a randomized controlled trial. Psychother. Psychosom.85, 218–228 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amelung, V., Nüsken, J. & Ledeganck, M. BMC-Innovationspanel.https://www.bmc.com/corporate/bmc-innovation-labs.html (2021).
- 13.Brönneke, J. B., Hagen, J., Kircher, P. & Matthies, H. Digitalisierte Gesundheitsversorgung im Jahr 2030 - ein mögliches Szenario. Bundesgesundheitsblatt Gesundheitsforsch. Gesundheitsschutz64, 1285–1291 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uncovska, M., Freitag, B., Meister, S. & Fehring, L. Patient acceptance of prescribed and fully reimbursed mHealth apps in Germany: An UTAUT2-based online survey study. J. Med. Syst.47, 14 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Firth, J. et al. Can smartphone mental health interventions reduce symptoms of anxiety? A meta-analysis of randomized controlled trials. J. Affect Disord.218, 15–22 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Lingg, M. & Lütschg, V. Health system stakeholders’ perspective on the role of mobile health and its adoption in the swiss health system: Qualitative study. JMIR Mhealth Uhealth8, e17315 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemkens, L. G. Nutzenbewertung digitaler Gesundheitsanwendungen - Herausforderungen und Möglichkeiten. Bundesgesundheitsblatt Gesundheitsforsch. Gesundheitsschutz64, 1269–1277 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahlhausen, F. et al. There’s an app for that, but nobody’s using it: Insights on improving patient access and adherence to digital therapeutics in Germany. Digit Health8, 20552076221104672 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morton, K. et al. Using digital interventions for self-management of chronic physical health conditions: A meta-ethnography review of published studies. Patient Educ. Couns.100, 616–635 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahlhausen, F. et al. Physicians’ attitudes toward prescribable mHealth apps and implications for adoption in Germany: Mixed methods study. JMIR Mhealth Uhealth9, e33012 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uncovska, M., Freitag, B., Meister, S. & Fehring, L. Rating analysis and BERTopic modeling of consumer versus regulated mHealth app reviews in Germany. NPJ Digit Med.6, 115 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt, L., Pawlitzki, M., Renard, B. Y., Meuth, S. G. & Masanneck, L. The three-year evolution of Germany’s digital therapeutics reimbursement program and its path forward. NPJ Digit Med.7, 139 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bundesinstitut für Arzneimittel und Medizinprodukte. DiGA-Verzeichnis.https://diga.bfarm.de/de/verzeichnis?type=%5B%5D (2024).
- 24.Krämer, R., Köhne-Volland, L., Schumacher, A. & Köhler, S. Efficacy of a web-based intervention for depressive disorders: Three-arm randomized controlled trial comparing guided and unguided self-help with waitlist control. JMIR Form. Res.6, e34330 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haaf, R., Machleid, F. & Köhler, S. Überblick über digitale Gesundheitsanwendungen mit Fokus Depression. Nervenheilkunde42, 430–437 (2023). [Google Scholar]
- 26.Haaf, R. et al. Wirksamkeit in Deutschland verfügbarer internetbasierter Interventionen für Depressionen - ein systematisches Review mit Metaanalyse. Nervenarzt95, 206–215 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bratan, T. et al. E-Health in Deutschland. Entwicklungsperspektiven und internationaler Vergleich.https://www.econstor.eu/handle/10419/251366 (2022).
- 28.Barros, P. P. et al. Assessing the Impact of Digital Transformation of Health Services.https://health.ec.europa.eu/system/files/2019-11/022_digitaltransformation_en_0.pdf (2019).
- 29.Zanaboni, P. et al. Methods to evaluate the effects of internet-based digital health interventions for citizens: systematic review of reviews. J. Med Internet Res.20, e10202 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iribarren, S. J., Cato, K., Falzon, L. & Stone, P. W. What is the economic evidence for mHealth? A systematic review of economic evaluations of mHealth solutions. PLoS One12, e0170581 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolasa, K. & Kozinski, G. How to value digital health interventions? A systematic literature review. Int. J. Environ. Res. Public Health17, 2119 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bates, D. W., Landman, A. & Levine, D. M. Health apps and health policy: What is needed? JAMA320, 1975–1976 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Owens, D. K., Qaseem, A., Chou, R. & Shekelle, P. High-value, cost-conscious health care: Concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann. Intern. Med.154, 174–180 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Powell, A. & Torous, J. A patient-centered framework for measuring the economic value of the clinical benefits of digital health apps: Theoretical modeling. JMIR Ment. Health7, e18812 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo, X. et al. Cost-effectiveness of mobile health-based integrated care for atrial fibrillation: Model development and data analysis. J. Med. Internet Res.24, e29408 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewkowicz, D., Wohlbrandt, A. M. & Bottinger, E. Digital therapeutic care apps with decision-support interventions for people with low back pain in Germany: Cost-effectiveness analysis. JMIR Mhealth Uhealth10, e35042 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abadie, F. & Boehler, C. Monitoring and Assessment Framework for the European Innovation Partnership on Active and Healthy Ageing (MAFEIP).https://publications.jrc.ec.europa.eu/repository/handle/JRC96205 (2015).
- 38.Boehler, C., De Graaf, G., Steuten, L., Yang, Y. & Abadie, F. Development of a web-based tool for the assessment of health and economic outcomes of the European innovation partnership on active and healthy ageing (EIP on AHA). BMC Med Inf. Decis. Mak.15, S4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukuria, C. et al. Cost-effectiveness of an improving access to psychological therapies service. Br. J. Psychiatry202, 220–227 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Hollinghurst, S. et al. Cost-effectiveness of cognitive–behavioural therapy as an adjunct to pharmacotherapy for treatment-resistant depression in primary care: Economic evaluation of the CoBalT Trial. Br. J. Psychiatry204, 69–76 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Zethraeus, N., Johannesson, M., Jönsson, B., Löthgren, M. & Tambour, M. Advantages of using the net-benefit approach for analysing uncertainty in economic evaluation studies. Pharmacoeconomics21, 39–48 (2003). [DOI] [PubMed] [Google Scholar]
- 42.Rommel, A., Bretschneider, J., Kroll, L. E., Prütz, F. & Thom, J. Inanspruchnahme psychiatrischer und psychotherapeutischer Leistungen. Individuelle Determinanten und regionale Unterschiede. J. Health Monit.2, 3–23 (2017).37168125 [Google Scholar]
- 43.Messner, E.-M., Probst, T., O’Rourke, T., Stoyanov, S. & Baumeister, H. mHealth applications: Potentials, limitations, current quality and future directions. In Digital Phenotyping and Mobile Sensing. New Developments in Psychoinformatics (eds. by Baumeister, H. & Montag, C.) (Springer International Publishing 2019), pp. 235–248.
- 44.Kolovos, S. et al. Cost effectiveness of guided internet-based interventions for depression in comparison with control conditions: An individual-participant data meta-analysis. Depress Anxiety35, 209–219 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahlert, M., Breyer, F. & Schwettmann, L. What You Ask is What You Get: Willingness-to-Pay for a QALY in Germany.https://ideas.repec.org/p/ces/ceswps/_4239.html (2013). [DOI] [PubMed]
- 46.Schwarzer, R. et al. Systematic overview of cost-effectiveness thresholds in ten countries across four continents. J. Comp. Eff. Res.4, 485–504 (2015). [DOI] [PubMed] [Google Scholar]
- 47.IQWiG. General Methods. Version 7.0.https://www.iqwig.de/en/about-us/methods/methods-paper/ (2023).
- 48.Gensorowsky, D., Witte, J., Batram, M. & Greiner, W. Market access and value-based pricing of digital health applications in Germany. Cost. Eff. Resour. Alloc.20, 25 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Groene, N. & Schneck, L. Covering digital health applications in the public insurance system: How to foster innovation in patient care while mitigating financial risks - evidence from Germany. Front Digital Health5, 1217479 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greiner, W. & Gensorowsky, D. DiGA-Report 2022.https://www.tk.de/resource/blob/2125136/dd3d3dbafcfaef0984dcf8576b1d7713/tk-diga-report-2022-data.pdf (2022).
- 51.Gräfe, V., Moritz, S. & Greiner, W. Health economic evaluation of an internet intervention for depression (deprexis), a randomized controlled trial. Health Econ. Rev.10, 19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.GKV Spitzenverband. Bericht des GKV-Spitzenverbandes über die Inanspruchnahme und Entwicklung der Versorgung mit Digitalen Gesundheitsanwendungen (DiGA-Bericht).https://www.gkv-spitzenverband.de/krankenversicherung/digitalisierung/kv_diga/diga.jsp (2023).
- 53.Meskendahl, D. & Bachmann, T. Bericht des Spitzenverbands Digitale Gesundheitsversorgung über die Marktentwicklung digitaler Gesundheitsanwendungen (DiGA-Report).https://digitalversorgt.de/wp-content/uploads/2024/01/DiGA-Report-2023-SVDGV.pdf (2023).
- 54.Kessler, R. C. et al. The epidemiology of major depressive disorder: Results from the national comorbidity survey replication (NCS-R). JAMA289, 3095–3105 (2003). [DOI] [PubMed] [Google Scholar]
- 55.Haji Ali Afzali, H., Karnon, J. & Gray, J. A critical review of model-based economic studies of depression: modelling techniques, model structure and data sources. Pharmacoeconomics30, 461–482 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Kolovos, S. et al. Model-based economic evaluation of treatments for depression: A systematic literature review. Pharmacoecon Open1, 149–165 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siebert, U. et al. State-transition modeling: A report of the ISPOR-SMDM modeling good research pactices task force-3. Value Health15, 812–820 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Caro, J. J., Briggs, A. H., Siebert, U. & Kuntz, K. M. Modeling good research practices-overview: A report of the ISPOR-SMDM modeling good research practices task force-1. Value Health15, 796–803 (2012). [DOI] [PubMed] [Google Scholar]
- 59.Husereau, D. et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. BMC Med.11, 80 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Husereau, D. et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: Updated reporting guidance for health economic evaluations. Value Health25, 3–9 (2022). [DOI] [PubMed] [Google Scholar]
- 61.Sonnenberg, F. A. & Beck, J. R. Markov models in medical decision making: A practical guide. Med Decis. Mak.13, 322–338 (1993). [DOI] [PubMed] [Google Scholar]
- 62.Nübel, J., Müllender, S., Hapke, U. & Jacobi, F. Epidemie der Depression? Prävalenzentwicklung und Inanspruchnahme von Hilfs- und Versorgungsangeboten. Nervenarzt90, 1177–1186 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Wittchen, H.-U. & Jacobi, F. Die Versorgungssituation psychischer Störungen in Deutschland. Bundesgesundheitsblatt Gesundheitsforsch Gesundheitsschutz44, 993–1000 (2001). [Google Scholar]
- 64.Messori, A. & Trippoli, S. The results of a pharmacoeconomic study: Incremental cost-effectiveness ratio versus net monetary benefit. Heart103, 1746 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Krummenauer, F. & Landwehr, I. Incremental cost effectiveness evaluation in clinical research. Eur. J. Med Res.10, 18–22 (2005). [PubMed] [Google Scholar]
- 66.Ulbrich, L. & Kröger, C. Monetary valuation of a quality-adjusted life year (QALY) for depressive disorders among patients and non-patient respondents: A matched willingness to pay study. Clin. Psychol. Eur3, e3855 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Statistisches Bundesamt. Gross domestic product (GDP).https://www.destatis.de/EN/Themes/Economy/National-Accounts-Domestic-Product/Tables/gdp-bubbles.html (2023).
- 68.Incerti, D. & Jansen, J. P. Hesim: Health economic simulation modeling and decision analysis. 10.48550/arXiv.2102.09437 (2021).
- 69.Baio, G. & Dawid, A. P. Probabilistic sensitivity analysis in health economics. Stat. Methods Med. Res.24, 615–634 (2015). [DOI] [PubMed] [Google Scholar]
- 70.Alarid-Escudero, F. et al. An introductory tutorial on cohort state-transition models in R using a cost-effectiveness analysis example. Med Decis. Mak.43, 3–20 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Briggs, A. H., Goeree, R., Blackhouse, G. & O'Brien, B. J. Probabilistic analysis of cost-effectiveness models: Choosing between treatment strategies for gastroesophageal reflux disease. Med. Decis. Mak.22, 290–308 (2002). [DOI] [PubMed] [Google Scholar]
- 72.Hunink, M. G. M. & Weinstein, M. C. Decision making in health and medicine. Integrating evidence and values 2nd edn, Vol. 446 (Cambridge University Press, Cambridge, 2016).
- 73.Carreras, G., Baccini, M., Accetta, G. & Biggeri, A. Bayesian probabilistic sensitivity analysis of Markov models for natural history of a disease: An application for cervical cancer. ijph9, 3 (2012). [Google Scholar]
- 74.Parmigiani, G. Measuring uncertainty in complex decision analysis models. Stat. Methods Med. Res.11, 513–537 (2002). [DOI] [PubMed] [Google Scholar]
- 75.Kligerman, M. P., Devine, E. E., Bentzley, J. P. & Megwalu, U. C. Cost-effectiveness of depression screening for otolaryngology-head and neck surgery residents. Laryngoscope131, 502–508 (2021). [DOI] [PubMed] [Google Scholar]
- 76.Grobe, T. G. et al. Prävalenzen von Depressionen bei Erwachsenen – eine vergleichende Analyse bundesweiter Survey- und Routinedaten. Gesundheitswesen81, 1011–1017 (2019). [DOI] [PubMed] [Google Scholar]
- 77.Statistisches Bundesamt. Population by nationality and sex (quarterly figures).https://www.destatis.de/EN/Themes/Society-Environment/Population/Current-Population/Tables/liste-current-population.html#616588 (2023).
- 78.Stahmeyer, J. T. et al. The state of care for persons with a diagnosis of depression. Dtsch Arztebl Int119, 458–465 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gräfe, K., Zipfel, S., Herzog, W. & Löwe, B. Screening psychischer Störungen mit dem “Gesundheitsfragebogen für Patienten (PHQ-D)”. Diagnostica50, 171–181 (2004). [Google Scholar]
- 80.Kroenke, K., Spitzer, R. L. & Williams, J. B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern Med.16, 606–613 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zuithoff, N. P. A. et al. The patient health questionnaire-9 for detection of major depressive disorder in primary care: Consequences of current thresholds in a crosssectional study. BMC Fam. Pr.11, 98 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bundesärztekammer, Kassenärztliche Bundesvereinigung & Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften. Nationale VersorgungsLeitlinie Unipolare Depression. Langfassung, Version 3.1.https://register.awmf.org/de/leitlinien/detail/nvl-005 (2022).
- 83.Whisman, M. A. & Richardson, E. D. Normative data on the beck depression inventory-second edition (BDI-II) in college students. J. Clin. Psychol.71, 898–907 (2015). [DOI] [PubMed] [Google Scholar]
- 84.Kocalevent, R.-D., Hinz, A. & Brähler, E. Standardization of the depression screener patient health questionnaire (PHQ-9) in the general population. Gen. Hosp. Psychiatry35, 551–555 (2013). [DOI] [PubMed] [Google Scholar]
- 85.Statistisches Bundesamt. Krankheitskosten: Deutschland, Jahre, Krankheitsdiagnosen (ICD-10), Geschlecht, Altersgruppen.https://www-genesis.destatis.de/genesis/online?sequenz=tabelleErgebnis&selectionname=23631-0003&sachmerkmal=ICD10Y&sachschluessel=ICD10-F32-F34&transponieren=true#abreadcrumb (2023).
- 86.Sonntag, M., König, H.-H. & Konnopka, A. The estimation of utility weights in cost-utility analysis for mental disorders: A systematic review. Pharmacoeconomics31, 1131–1154 (2013). [DOI] [PubMed] [Google Scholar]
- 87.Whitehead, S. J. & Ali, S. Health outcomes in economic evaluation: The QALY and utilities. Br. Med Bull.96, 5–21 (2010). [DOI] [PubMed] [Google Scholar]
- 88.Deutsche Bundesbank. Discount Interest Rates. https://www.bundesbank.de/en/statistics/money-and-capital-markets/interest-rates-and-yields/discount-interest-rates/discount-interest-rates-793422 (2023).
- 89.Statistisches Bundesamt. Bevölkerung Nach Altersgruppen (ab 2011).https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Bevoelkerung/Bevoelkerungsstand/Tabellen/liste-altersgruppen.html#474508 (2023).
- 90.Statistisches Bundesamt. Sterbefälle - Fallzahlen nach Tagen, Wochen, Monaten, Altersgruppen, Geschlecht und Bundesländern für Deutschland 2016-2023.https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Bevoelkerung/Sterbefaelle-Lebenserwartung/Tabellen/sonderauswertung-sterbefaelle.html (2023).
- 91.Meyer, B. et al. Effects of an Internet intervention (Deprexis) on severe depression symptoms: Randomized controlled trial. Internet Interventions2, 48–59 (2015). [Google Scholar]
- 92.Moritz, S., Schilling, L., Hauschildt, M., Schröder, J. & Treszl, A. A randomized controlled trial of internet-based therapy in depression. Behav. Res. Ther.50, 513–521 (2012). [DOI] [PubMed] [Google Scholar]
- 93.Moritz, S. et al. Effects of online intervention for depression on mood and positive symptoms in schizophrenia. Schizophr. Res.175, 216–222 (2016). [DOI] [PubMed] [Google Scholar]
- 94.Beiwinkel, T., Eißing, T., Telle, N.-T., Siegmund-Schultze, E. & Rössler, W. Effectiveness of a web-based intervention in reducing depression and sickness absence: Randomized controlled trial. J. Med. Internet Res.19, e213 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baumeister, A. & Moritz, S. Studienbericht Novego Depression.https://www.novego.de/wp-content/uploads/2023/10/20230907_Studienbericht-Novego-Depression-final.pdf (2023).
- 96.Bundesinstitut für Arzneimittel und Medizinprodukte. Arzneimittel-Festbeträge.https://www.bfarm.de/DE/Arzneimittel/Arzneimittelinformationen/Festbetraege-und-Zuzahlungen/Festbetraege/_node.html (2023).
- 97.Wissenschaftliches Institut der AOK. PharMaAnalyst. Verordnungsdaten 2022.https://arzneimittel.wido.de/PharMaAnalyst/?1 (2022).
- 98.IGES Institut. Arzneimittelatlas: Die bedeutendsten Mittel mit stimulierender Wirkung auf das Zentralnervensystem (Psychoanaleptika). N06 Psychoanaleptika.https://www.arzneimittel-atlas.de/arzneimittel/n06-psychoanaleptika/top-10/ (2022).
- 99.Kassenärztliche Bundesvereinigung. Einheitlicher Bewertungsmaßstab (EBM).https://www.kbv.de/html/ebm.php (2023).
- 100.GKV Spitzenverband. Psych-Entgeltsystem. PEPP 2023.https://www.gkv-spitzenverband.de/krankenversicherung/krankenhaeuser/psychiatrie/pepp_entgeltsystem_2023/pepp_5.jsp (2023).
- 101.Statista. Durchschnittliche Verweildauer in deutschen Krankenhäusern nach medizinischer Fachabteilung im Jahr 2021.https://de.statista.com/statistik/daten/studie/369355/umfrage/verweildauer-in-deutschen-krankenhaeusern-nach-medizinischen-fachabteilungen/ (2021).
- 102.Osterloh, F. Psychische Erkrankungen: Hohe Rehospitalisierungsrate. Aerzteblatt10, 347–348 (2011). [Google Scholar]
- 103.Mohiuddin, S. & Payne, K. Utility values for adults with unipolar depression: Systematic review and meta-analysis. Med. Decis. Mak.34, 666–685 (2014). [DOI] [PubMed] [Google Scholar]
- 104.Kolovos, S. et al. Utility scores for different health states related to depression: Individual participant data analysis. Qual. Life Res.26, 1649–1658 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Revicki, D. A. & Wood, M. Patient-assigned health state utilities for depression-related outcomes: Differences by depression severity and antidepressant medications. J. Affect Disord.48, 25–36 (1998). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The code that supports the findings of this study is available from the corresponding author upon reasonable request.