Abstract
Primary open-angle glaucoma typically presents as two subtypes. This study aimed to elucidate the shared and distinct genetic architectures of normal-tension (NTG) and high-tension glaucoma (HTG), motivated by the need to develop intraocular pressure (IOP)-independent drug targets for the disease. We conducted a comprehensive multi-ethnic meta-analysis, prioritized variants based on functional annotation, and explored drug-gene interactions. We further assessed the genetic overlap between NTG and HTG using pairwise GWAS analysis. We identified 22 risk loci associated with NTG, 17 of which have not previously been reported for NTG. Two loci, BMP4 and TBKBP1, have not previously been associated with glaucoma at the genome-wide significance level. Our results indicate that while there is a significant overlap in risk loci between tension subtypes, the magnitude of the effect tends to be lower in NTG compared to HTG, particularly for IOP-related loci. Additionally, we identified a potential role for biologic immunomodulatory treatments as neuroprotective agents.
Subject terms: Optic nerve diseases, Genome-wide association studies, Drug discovery
This study investigates the genetic similarities and differences between two subtypes of glaucoma (normal tension and high tension). Multi-ethnic meta-analysis reveals overlapping risk loci, with a lower effect magnitude in normal tension glaucoma. The authors also use their gene discovery approach to highlight possible neuro-protective drug targets for glaucoma.
Introduction
Primary open-angle glaucoma (POAG) is a chronic and progressive optic neuropathy that is characterized by damage to the optic nerve and loss of vision1. It is a leading cause of irreversible blindness, and its worldwide prevalence was estimated to be around 2.4% in 20212. POAG is one of the most heritable diseases3 and typically presents as two subtypes: high-tension glaucoma (HTG) and normal-tension glaucoma (NTG).
HTG is defined as POAG in patients with intraocular pressure (IOP) ≥ 21 mmHg, and it is usually associated with the ocular hypertension process, in which high IOP is the main risk factor in the disease progression4. NTG is defined as POAG in patients with IOP consistently measured <21 mmHg, and is believed to be more related to specific processes such as vascular dysregulation and ischemia5,6. However, the stratification of POAG by IOP is controversial whereby different tension subtypes may be part of the same pathogenic process7 versus the claim that primary neurodegeneration features are more prominently in NTG8.
The current treatments for NTG/HTG aim to lower IOP, which can slow disease progression9,10. Yet, over 50% of POAG cases are not diagnosed until there is irreversible optic nerve damage11. This highlights the importance of developing more accurate screening methods to identify individuals at high risk for NTG earlier in the disease process. In addition, a deeper understanding of the genetics of the neurodegenerative process of glaucoma could help develop neuroprotective treatments, which are currently unavailable12, by inhibiting the pathogenic cascade that leads to nerve damage.
In this work, we aimed to untangle the shared and specific genetic architecture between the glaucoma tension subtypes and identify specific loci for NTG, which could lead to a more accurate risk assessment and screening prioritization. We also used our gene-discovery approach to highlight possible neuro-protective drug targets for POAG.
Results
Multitrait meta-analysis
Throughout our study, we used a two-stage meta-analysis approach. The first stage included European data from the IGGC, UK Biobank, CLSA, and VCDR. Upon validating the initial results on Asian participants from IGGC and Europeans using Finngen data, the second stage incorporated these datasets along with the results from the first stage.
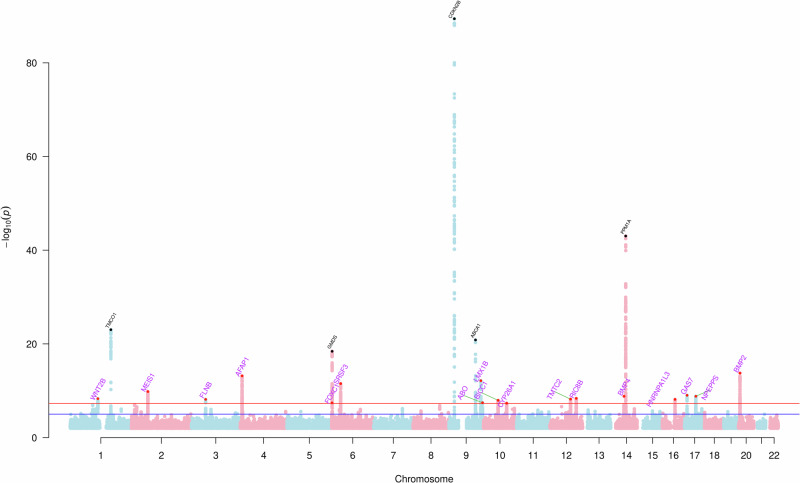
This study identified 22 independent loci associated with NTG by meta-analyzing multiple ethnicities and traits (Fig. 1). The first stage MTAG analysis found 11 genome-wide significant risk loci, nine of which replicated after Bonferroni correction for multiple testing (P < 0.05/11) and the remaining two at nominal significance (P < 0.05) in Finngen; see Supplementary Data 1. In the IGGC Asian meta-analysis, three were replicated after Bonferroni correction and five at nominal significance (Supplementary Data 1). Three loci did not replicate in IGGC Asians (rs6054248, rs57831033, and rs6539772), and two, rs66998222 and rs2971831, did not replicate after correction for multiple testing in FinnGen, however, all of them maintained the same direction of effect. The resulting meta-analysis demonstrated low genetic inflation (λGC = 1.08; see Q–Q plot in Supplementary Fig. 1), an LDSC intercept of 1.02 (SE = 0.007), and an array heritability estimate of 0.22 (SE = 0.05) for NTG in Europeans. We further validated the consistency of the results between stage one and stage two of the meta-analysis using an IVW approach (r = 0.99, P = 1.7e-11); as per Supplementary Fig. 2. Examination of all the loci across the genome using the GWAS pairwise method (GWAS-PW) indicated that the risk loci are shared across NTG and HTG (PPA > 0.8), consistent with the high genetic correlation (rg) between HTG and NTG (rg = 0.84, se = 0.07 P = 5.1e−33).
Fig. 1. Manhattan plot based on the meta-analysis of normal tension glaucoma (NTG).
Each dot (N = 22) represents a single nucleotide polymorphism (SNP), and the red line represents the threshold for multiple testing correction (p < 5 × 10−8) and blue line p < 5 × 10−6; p-values derived from logistic regression models are two-sided. Previously unidentified loci are highlighted in purple and represented in the plot as red dots, while known loci are in black.
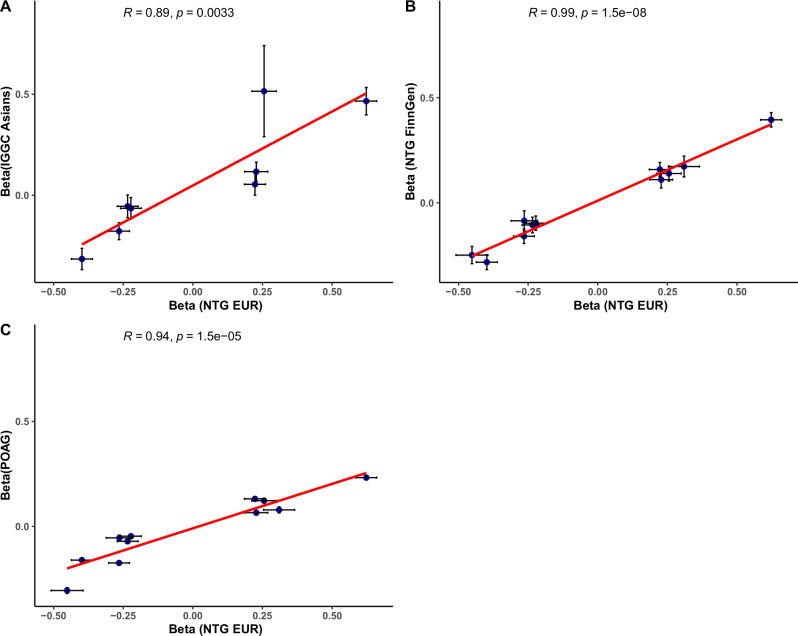
For the genome-wide significant NTG loci from our stage-one model, we found a high correlation (r = 0.89, P = 0.003) between the effect size on NTG for the European discovery sample datasets and the effect sizes estimated in the IGGC Asian NTG datasets (Fig. 2A). The correlation was even higher (r = 0.99, P = 1.5e−8) when comparing the European discovery sample NTG datasets and NTG in FinnGen (Fig. 2B). We further tested the correlation of effect sizes between the MTAG-independent NTG genome-wide significant loci and the published IGGC POAG GWAS, which revealed a lower correlation than the results obtained from NTG in Finngen (Fig. 2C). Similarly, the correlation between the effect sizes of the risk loci associated with POAG13 and NTG in FinnGen was smaller (r = 0.53, P < 0.001); as shown in Supplementary Fig. 3.
Fig. 2. Correlation of the allele effect estimates using a two-sided linear model between multitrait analysis of European ancestry (EUR) and two independent cohorts, FinnGen and the Asians in the International Genetics of Glaucoma Consortium (IGGC).
Effects estimate (Beta) are presented as center points and black crosses represent 95% confidence intervals. A Correlation of the effect estimates between the NTG of EUR and IGGC Asians (N = 8), B NTG of EUR and FinnGen (N = 11) and C NTG of EUR and POAG (N = 11).
The second stage of the meta-analysis across ethnicities (i.e., joint analysis of the discovery and validated datasets from stage 1) found 22 independent genome-wide significant loci associated with NTG (Supplementary Data 2), 17 have not been identified for NTG and two loci (MIR5580/BMP4 and KPNB1/TBKBP1) have not been associated at the genome-wide significant level with any glaucoma subtype. However, some of these loci have been previously associated at a genome-wide significant level with primary open-angle glaucoma (16/22), high-tension glaucoma (7/22), and some glaucoma endophenotypes such as IOP (9/22) and VCDR (21/22); VCDR has been previously associated with MIR5580 and KPNB1.
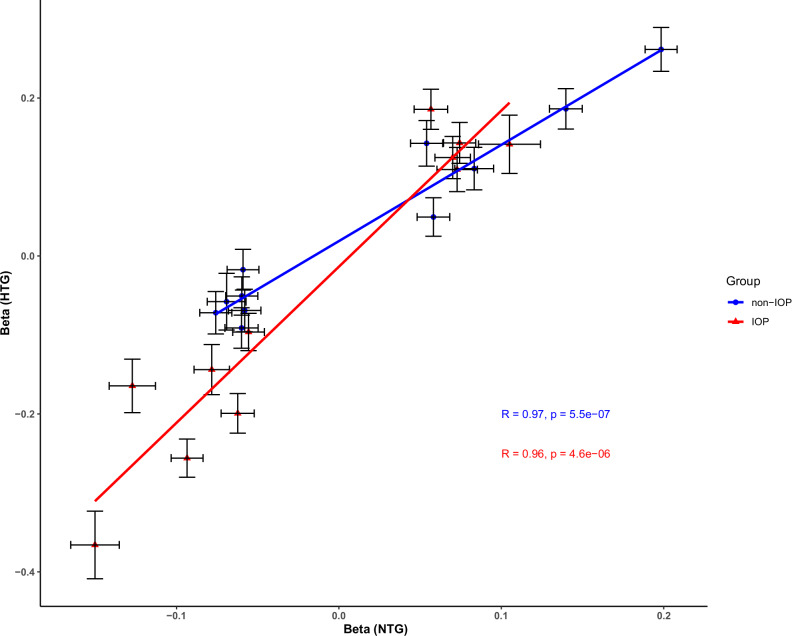
The effect size of the genome-wide significant loci tends to be lower in NTG when compared with HTG (Fig. 3); on average, the HTG effects on the log(OR) scale were 1.6 times higher (SE = 0.13) based on an inverse variance weighting regression of the effect sizes. This difference was larger for IOP loci (P < 0.05 for IOP), with the HTG effects being 1.9 times higher (SE = 0.2) than NTG compared with non-IOP loci (P > 0.05 for IOP) where the HTG effects were only 1.2 times higher (SE = 0.09). Seven loci showed statistically significantly different magnitudes of effect between NTG and HTG after multiple testing corrections (P < 0.002; 0.05/22 loci), as shown in Supplementary Data 2. Four loci (rs2472493, nearest gene is ABCA1, rs12602519, nearest gene is GAS7, rs4657477, nearest gene is TMCO1, and rs2745572, nearest gene is FOXC1) were consistently using SNP effect sizes from clinically diagnosed NTG in FinnGen and IGGC.
Fig. 3. Inverse weighted variance (IVW) correlation between the effect sizes (N = 22), estimated in the log(OR), of normal tension glaucoma (NTG) and high tension glaucoma (HTG).
The slope for IOP loci (P < 0.05 for IOP; red line) shows that the effect sizes on average are 1.9 times higher in HTG compared to NTG whereas the slope for non-IOP loci (P > 0.05 for IOP; blue line), shows that the effect sizes on average are more similar (only 1.2 times higher in HTG); P-values derived from IVW are two-sided. Effects estimate (Beta) are presented as center points and black crosses represent 95% confidence intervals.
Functional annotation and drug-gene interaction
Gene-based analysis in mBAT-combo on 18,766 genes identified 25 genes associated with NTG (P < 0.05/18,766) (Supplementary Data 3), and gene-set enrichment analysis in MAGMA identified a biological process “go_response_to_laminar_fluid_shear_stress” that is likely associated with the etiology of NTG (P < 2.7e−6). Although gene-based analysis did not identify additional risk loci over and above the SNP-based analysis, it provided gene-based support for the association of genes within the two loci (MIR5580/BMP4 and KPNB1/TBKBP1/EFCAB13), which were not previously associated at the genome-wide significant level with any glaucoma phenotype. The results of the conditional analysis in GCTA-COJO for the top SNP of KPNB1 (rs7220935) and EFCAB13 (rs9894179) suggest that this could be part of the same signal at the TBKBP1 locus, as the signal for TBKBP1 did not remain in the conditional analysis. Similarly, the signal for MIR5580 did not remain after the analysis was conditioned for BMP4, indicating that it is likely the same locus, as shown in Supplementary Figs. 12–15. KPNB1 has been previously associated with snoring14, hemoglobin levels15, and Body Mass Index (BMI)16.
Single-cell expression results showed a statistically significant association between the expression of BMP4 and NTG in serotonin transporter neurons that were exposed to rotenone-induced oxidative stress. Expression of two other genes, ALDH9A1 and MGST3, were also associated with NTG in neuron or neuron-like cells; however, these associations were nominally significant or had a HEIDI P < 0.05, likely depicting heterogeneity in the association; as per Supplementary Data 5. Results for BMP4 were also consistent with multi-omics analysis where MIR5580/BMP4 (rs12893484) was associated with methylation at a DNA promoter region (cg16720578), with a posterior probability of association greater than 0.9 for a combined effect of changes in gene expression and methylation of BMP4 in development of NTG. Results from eQTL on peripheral blood using the SMR framework also found an association with NTG and 6 genes, as per the supplementary Data 6, including TBKBP1, however, only ITGB3 association remained significant after assessing for heterogeneity (P > 0.05 [HEIDI]). We further assessed the expression profile using retinal eQTL data, but the results were inconclusive, likely due to a lower sample size of the retinal eQTL data.
Results from DBIbd found interactions between four genes (i.e., ABCA1, CDKN2A, CDKN2B, and ITGB3) and 42 drugs, as shown in Supplementary Data 4. Of interest, CDKN2A, CDKN2B, and ITGB3, are genes that have been previously associated with the neurodegeneration of the optic nerve and had the majority of the drug-gene interactions (36/42). Highlighting a potential role for biologic immunomodulatory treatments as neuroprotective agents; as per Supplementary Data 4.
Discussion
We conducted a GWAS and identified 22 independent loci associated with NTG, thereby expanding our understanding of the genetic architecture underlying the condition. Gene enrichment analysis and SNP-based tests identified two loci, BMP4 and TBKBP1, which had not previously been associated at a genome-wide significant level with any glaucoma subtype.
TBKBP1 is an adaptor protein that binds to TANK-binding kinase 1 (TBK1), a gene known to be involved in various cellular processes, including inflammation, autophagy, and the innate immune response17. Previous studies have shown an association between TBK1 mutations and NTG, where gene duplications and triplications have been associated with early-onset familial NTG1,18,19. Consistent with these studies, our results highlight an association between TBKBP1 in peripheral blood and NTG. These findings support the potential involvement of the adaptor protein TBKBP1 in the development of NTG.
Through this study, we also found an association between BMP4 and NTG, an association that was confirmed through multi-omics analysis. Bone Morphogenetic Protein 4 (BMP4) has been associated with retinal ganglion cell integrity in mouse models20. There is a well-known interaction between BMP4 and TGF-β that could play a significant role in NTG pathogenesis through effects on the trabecular meshwork and optic nerve head21,22. TGF-β2 is significantly upregulated in patients with glaucoma21, and increased concentrations of TGF-β have been observed in the aqueous humor and reactive optic nerve astrocytes of individuals diagnosed with POAG23. It has also been suggested that BMP4 acts as an endogenous inhibitor of TGF-β2 within the human trabecular meshwork and aids in controlling the increased accumulation of extracellular matrix protein deposits24, thereby maintaining IOP within normal limits.
Our European meta-analysis revealed a consistent loci effect when compared to two independent cohorts, Finngen and the Asian meta-analysis of IGGC. Although our first stage meta-analysis used proxy phenotype information for NTG (based on IOP < 21 mmHg, as well as VCDR), the results replicated well in IGGC and Finngen with clinically diagnosed NTG. Notably, three loci were not present in the Asian population, one of which corresponded to the TMCO1 region where the key SNP (rs4657477) is monomorphic in Asians. Given the higher prevalence of NTG in Asian populations25, the absence of a TMCO1-related variant could be expected as this gene is likely associated with higher IOP26.
Genes such as FOXC1 are likely to have a primary effect on IOP but also a secondary effect on VCDR, as a result of the pressure effect. In our study, we adjust for IOP to focus our efforts on identifying loci that act on VCDR in a way that is relatively unaffected by IOP. In the second stage of the meta-analysis, we sought to leverage the effect of known variants in genes such as CDKN2BAS and SIX1, which affect VCDR with little or no impact on IOP, across the genome. These results still show a high genetic correlation between HTG and NTG, with shared risk loci across the genome. However, further analysis revealed that NTG loci could be divided into two groups, ‘neurodegenerative-driven’ (VCDR-related) and ‘IOP-driven’. Genes in the IOP-driven group (e.g., TMCO1, GAS7, ABCA1) had a much larger effect on HTG than on NTG, while genes in the neurodegenerative-driven group (e.g., SIX6/BICC1, CDKN2BAS, MTMC2, CASC20) had a similar effect on both HTG and NTG risk. This suggests that the observed 1.6-fold increased effect on HTG compared to NTG is a result of a combination of two types of genetic variants, where the neurodegenerative-driven loci have a similar effect size on both HTG and NTG, while the IOP-driven loci have a much larger effect size on HTG than on NTG. Future studies of NTG may be able to leverage this further to estimate more accurate effect sizes, which could benefit some types of analysis, such as polygenic risk scores.
The genetic analysis of drug-gene interactions revealed a significant interaction between four genes and several drugs. Specifically, ABCA1 had an interaction with some cholesterol-lowering medications such as Probucol, Simvastatin, Pravastatin, and Atorvastatin. These drugs have been studied for their potential neuroprotective effects in animal models of glaucoma, and some have been found to lower IOP and improve retinal ganglion cell survival27,28. However, no drug-gene interactions associated with the neurodegeneration of the optic nerve (CDKN2A, CDKN2B and ITGB3) have been specifically proposed or studied as treatments for glaucoma and further research is necessary to evidence if these could be potential targets for neuroprotective treatments.
It is important to highlight that our results were limited by statistical power to identify loci with a small effect, warranting larger studies to identify such loci. We have shown that the VCDR-driven loci are shared across NTG and HTG, with similar effect sizes. For the IOP-driven loci, the largest ones affect NTG, although the effect size on NTG for such loci is much smaller than it is on HTG. Additionally, while our results were replicated in Asians, where NTG is more prevalent, further research is needed to validate our proposed explanations in other ethnic groups.
A limitation of our study is the reliance on IOP measurements, which included participants who had tonometry up to five years before or after the diagnosis of glaucoma. This introduces a risk of misclassification, particularly given that glaucoma diagnoses were self-reported in CLSA. While our two-stage approach to analysis mitigates some of these concerns by reducing the likelihood of false positives, it may increase the heterogeneity of the sample and the potential for false negatives. Consequently, while the loci identified in this study are likely robust, additional loci associated with NTG may remain undetected.
To conclude, our study identified 22 independent loci associated with NTG and revealed a high genetic correlation between NTG and HTG. We also found an association between two genes, NTG, BMP4, and TBKBP1, that have not been previously associated at a genome-wide level with any type of glaucoma. This highlights an important consistent association of the TANK-binding kinase 1 (TBK1) in the development of NTG and a role of BMP4 in NTG etiology, as underlined by our multi-omic analysis.
Despite the high genetic overlap, we have shown that IOP-related loci tend to have smaller effect sizes for NTG compared to HTG while, loci that are independent of IOP have similar effect sizes for NTG and HTG. This suggests that, even though shared mechanisms may lead to the development of major POAG subtypes, certain genetic profiles may be more sensitive to the ocular hypertension process. However, the presence of specific loci in NTG that are not associated with HTG at a genome-wide significant level might imply the existence of distinct mechanisms that primarily contribute to neurodegeneration, leading to ocular neuropathy and that are exacerbated by a comorbid increase in ocular pressure.
Methods
Study design and participants
To identify risk loci specific to NTG, we conducted a large multi-trait meta-analysis of genome-wide association studies (GWAS) across individuals of European and Asian descent. The European ancestry NTG data included the International Genetics of Glaucoma Consortium (IGGC; 3247 cases and 47,997 controls), UK Biobank (UKB; 2184 cases and 7000 controls), Canadian Longitudinal Study on Aging (CLSA; 755 cases and 3000 controls), FinnGen (Release 8, 1756 cases and 326,434 controls), and a structural measurement of the integrity of the optic nerve, vertical cup-to-disk ratio (VCDR, N = 97,939 participants). VCDR estimates were obtained from UKB and CLSA participants of European ancestry29. The Asian NTG data included a GWAS meta-analysis of 4418 cases and 34,303 controls from Hong Kong, Singapore, and Japan.
Glaucoma was self-reported in CLSA following diagnosis by a clinician as per the report in the item ‘ICQ_GLAUC_COM’ (Has a doctor ever told you that you have glaucoma?)30,31. UK Biobank was restricted to glaucoma diagnosed under the International Classification of Diseases (ICD) 10 criteria, without specific classification for HTG or NTG. Thus, participants with a diagnosis of glaucoma and IOP under 21 mmHg were considered NTG cases in UKB and CLSA, provided that the tonometry to measure IOP was performed within 5 years of the glaucoma diagnosis. However, to ensure consistency in results when IOP measurements are taken closer to the time of glaucoma diagnosis, we re-ran the analysis to include participants who had tonometry within one year of their diagnosis. The results were consistent, but the power decreased due to a reduction in the number of ‘probable NTG’ participants by approximately 4.5-fold, as shown in Supplementary Fig. 16. Maximum IOP was used when multiple measurements were present. Patients receiving glaucoma medication or those who had undergone surgery to decrease the IOP at the time of the tonometry were excluded from the analysis in UKB and CLSA. Controls were randomly selected from UKB and CLSA datasets, maintaining a ratio of 1:3, from a pool of individuals who had no reported ocular conditions. Given that UKB and CLSA cases were not clinically diagnosed with specificity for tension subtypes, we considered them as “probable NTG cases” and used them as a separate phenotype in the multi-trait meta-analysis. NTG was clinically diagnosed (in person or confirmed with medical records) in IGGC and FinnGen. Detailed information regarding recruitment, genotyping, and quality control are described within their respective publications32,33.
Genome-wide association studies (GWAS)
GWAS analysis was performed using UKB and CLSA participants of European ancestry determined through principal component analysis. We used Regenie v 2.3.4, a machine-learning method34, to run the GWAS. We included sex, age, and 10 principal components as covariates in the model. We meta-analyzed the UKB and CLSA results using METAL, a software to meta-analyze genome-wide association scans35. For IGGC NTG and VCDR data, we used the previously published GWAS summary statistics13,29. The VCDR GWAS was adjusted for IOP using the mtCOJO, a method to adjust estimates for the effect of another trait using GWAS summary statistics. For FinnGen, we used the release 8 normotensive glaucoma GWAS (https://r8.finngen.fi/pheno/H7_GLAUCOMA_NTG).
Multitrait meta-analysis
We used multi-trait analysis of GWAS (MTAG), version 2020080, a method for the joint analysis of summary statistics from genome-wide association studies (GWAS) of correlated traits36, and an inverse variance weighting meta-analysis approach through METAL, version 20211102, to enhance the power for discovering NTG risk loci. MTAG leverages the genetic correlation between correlated traits to boost the statistical power for the GWAS of each input trait. We conducted a two-stage multi-trait meta-analysis.
The first stage entailed a meta-analysis using METAL, incorporating NTG probable cases from the UKB and CLSA. Subsequently, the results of the UKB and CLSA meta-analysis, vertical cup-disc ratio (VCDR) adjusted for intraocular pressure (IOP), and clinically diagnosed NTG cases of European ancestry from the International Glaucoma Genetics Consortium (IGGC) were integrated into a multi-trait meta-analysis using MTAG. This first stage aimed to enhance the statistical power of IGGC NTG cases of European ancestry.
Loci identified as independent and genome-wide significant (P < 5e−8) in the first-stage analysis were validated using an inverse variance weighting approach in independent datasets (i.e., FinnGen and Asian IGGC). Upon validation of the first-stage results, we jointly analyzed phenotypes that were clinically diagnosed (i.e., IGGC in Europeans and Asians, FinnGen) using METAL and then used MTAG to incorporate the probable NTG cases of UKB and CLSA, and IOP-adjusted VCDR. The second stage resulted in an overall sample size of 7942 cases and 384,431 controls, with VCDR values available for 97,939 participants. VCDR data from UKB and CLSA were used for their high correlation with the clinically diagnosed phenotype, aiming to boost the statistical power of the associations.
To compare HTG and NTG, we used the HTG GWAS published by Gharahkhani et al. 13 based on 5144 HTG cases and 76,997 controls. We further compared the magnitude of the effect of the risk loci between NTG and IOP26, vertical cup-to-disk ratio29 and POAG13. The difference in magnitude of effect between the MTAG NTG results and previously published results for HTG and between FinnGen NTG and HTG was estimated using the equation below. Given the strong correlation between the two phenotypes, we decided to include the method described in Randall et al. 37, which accounted for differences between NTG and HTG effect estimates , and corresponding standard errors , using t statistics and adjusting for HTG and NTG correlation, where r was computed as the genetic correlation coefficient across all the loci; effects were estimated on the log(OR) scale:
| 1 |
The genetic correlation between HTG and NTG was evaluated using linkage disequilibrium score regression (LDSC). LDSC is a technique that estimates the genetic correlation between phenotypes by analyzing GWAS summary statistics, while also taking into consideration factors such as overlapping samples and polygenicity38. We used the 1000 Human Genome Project reference panel for LDSC estimations. Additionally, LDSC was utilized to estimate the LD-Score intercept and heritability of NTG in individuals with European ancestry.
We then compared differences in the genetic architecture of NTG and HTG using the GWAS pairwise method (GWAS-PW)39. GWAS-PW is a method that evaluates the genetic overlap over specific genomic regions by splitting the genome into 1703 segments and estimating the posterior probability of four different models: (1) the region is unique to NTG, (2) unique to HTG, (3) shared with both with a common causal variant and (4) shared with both without a common causal variant.
Functional annotation and drug–gene interaction
To improve our power to detect genes associated with NTG, we employed a Gene-based association test, mBAT-combo v 1.94.1, a method that is considered robust to detect SNPs with masking effects40. We corrected for multiple testing using a Bonferroni approach given by the number of genes tested in the analysis (α = 0.05/18,766 [genes], P < 2.7e−06). We further performed conditional analysis using GCTA-COJO v 1.91.7 to identify independent genetic signals in low linkage disequilibrium41. To find biological pathways associated with NTG, we used gene-set tests in the program Meta-Analysis Gene-set Mining of GWAS (MAGMA) v 1.08. We applied Bonferroni correction for multiple testing, accounting for the total number of gene-sets tested (α = 0.05/15,483 [gene-sets], P < 3.3e−06).
We then leveraged omics data to explore the functional relevance of the genes identified in the mBAT-combo gene-based analysis. First, we used summary-data-based Mendelian randomization (SMR) (Zhu et al.42), v 1.3.1, to identify putative causal associations between gene expression and NTG based on eQTL data from peripheral blood of 2765 individuals from the Consortium for the Architecture of Gene Expression (CAGE)43 and retinal eQTL data from 453 individuals44. We then conducted an in-depth analysis of cell-type specific transcriptomic profiles in retinal ganglion cells, the most relevant cells implicated in the development of NTG. This analysis employed single-cell RNA-sequencing data derived from 23 distinct sub-populations of retinal ganglion cells, which collectively constituted a sample comprising 247,520 cells45 and over one million neuron-like cells, including cells that had been exposed to rotenone to induced oxidative stress46. These neurons encompassed a diverse range, including dopaminergic neurons, serotonin transporters, astrocyte-like cells, ependymal cells, and cell clusters undergoing neuronal differentiation. For the assessment of gene expression across multiple cell lines in relation to NTG, we employed the SMR method. Additionally, to account for multiple testing, we applied the Bonferroni correction technique, considering the effective number of independent genes being analyzed (α = 0.05/25 [genes], p < 0.002).
We further assessed the multi-omic profile of genes that were consistent between the gene-based and sc-RNA Seq-based approach through the Omics Pleiotropic Association (OPERA), v 1.0.0. OPERA is a Bayesian method that integrates the SMR and HEIDI approach for multi-omics analysis, aiming to provide further interpretation of the biological mechanisms underlying GWAS signals and to prioritize molecular phenotypes47. This analysis incorporates the single-cell RNA-sequencing data, described in the previous paragraph, methylation profile based on mQTL of peripheral blood samples from 1980 participants48 and eQTL data from the peripheral blood of 2765 individuals from the Consortium for the Architecture of Gene Expression (CAGE)43.
Genes that reached genome-wide significance in the mBAT-combo gene-based tests were included for drug–gene interaction using the Drug–Gene Interaction Database (DBIbd) v 4.049 to identify genes that could be prioritized for targeted therapy or drug development. DBIbd is a scientifically curated database that provides information on known and predicted interactions between drugs and genes. It integrates data from multiple sources to provide a comprehensive overview of drug–gene interactions and is commonly used as a resource for evaluating the potential impact of genetic variation on drug response.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
P.G. is supported by an NHMRC Investigator Grant (#1173390). S.M. is supported by an NHMRC Senior Research Fellowship and an NHMRC Program Grant (APP1150144). J.L.W. is supported by NIH/NEI R01 EY022305 and P3O EY014104. We acknowledge the donors of the National Glaucoma Research, a program of the BrightFocus Foundation, for support of this research (BrightFocus Grant Submission Number: G2021009S). L.R.P. acknowledges grant funding from Research to Prevent Blindness (NYC), The Glaucoma Foundation (NYC), and NEI (R01 EY032559 and EY036460). I would like to acknowledge the International Glaucoma Genetics Consortium (IGGC) for their invaluable contribution to this paper. Their provision of data, as well as the insightful suggestions and comments from multiple members, has significantly enriched the quality and depth of our research. The opinions expressed in this manuscript are the author’s own and do not reflect the views of the CLSA or any affiliated institution. This research was made possible using the data/biospecimens collected by the CLSA. Funding for the CLSA is provided by the Government of Canada through the Canadian Institutes of Health Research (CIHR) under grant reference: LSA 94473 and the Canada Foundation for Innovation, as well as the following provinces, Newfoundland, Nova Scotia, Quebec, Ontario, Manitoba, Alberta, and British Columbia. This research has been conducted using the CLSA dataset [Baseline Comprehensive Dataset version 4.0, Follow-up 1 Comprehensive Dataset version 1.0 and Genome-wide Genetic Data Version 3.0], under Application Number 190225. The CLSA is led by Drs. Parminder Raina, Christina Wolfson, and Susan Kirkland. This work was conducted using the UK Biobank Resource (application number 25331). The UK Biobank was established by the Wellcome Trust medical charity, the Medical Research Council United Kingdom, the Department of Health United Kingdom, the Scottish Government, and Northwest Regional Development Agency. It also had funding from the Welsh Assembly Government, the British Heart Foundation, and the Diabetes United Kingdom. The eye and vision dataset has been developed with additional funding from The NIHR Biomedical Research Centre at Moorfields Eye Hospital and the UCL Institute of Ophthalmology, Fight for Sight Charity United Kingdom, Moorfields Eye Charity United Kingdom, The Macula Society United Kingdom, The International Glaucoma Association United Kingdom and AlconResearch Institute (USA). APK is supported by a UK Research and Innovation Future Leaders Fellowship, an Alcon Research Institute Young Investigator Award and a Lister Institute for Preventive Medicine Award.
Author contributions
P.G. and S.M. conceived the study and designed the analyses. S.D.-T. carried out the analyses with support from W.H. and wrote the manuscript with input and support from R.Y., A.P.K., C.J.H., P.G.H., L.R.P., Y.W., M.K., M.A., T.A., C.-Y.C., C.C.K., P.K., J.H.K., A.W.H., D.A.M., J.E.C., J.L.W., and J.-S.O.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The GWAS summary data for the meta-analysis generated in this study is available on Zenodo [10.5281/zenodo.14010557]. Individual-level data from the UK Biobank were accessed under application number 25331, and CLSA data under application number 190225. Researchers may obtain access to these datasets by applying directly to each cohort: UK Biobank (https://www.ukbiobank.ac.uk/) and CLSA. GWAS data from the IGGC in European and Asian populations used in this study are available upon request to the corresponding authors of Gharahkhani et al. 13; the POAG GWAS data used in this study can be accessed via the GWAS Catalog (GCST90011766). FinnGen normotensive glaucoma GWAS data used in this study is accessible at https://r8.finngen.fi/pheno/H7_GLAUCOMA_NTG. The GWAS summary statistics for vertical cup-disc ratio (VCDR) GWAS used in this study are available at https://xikunhan.github.io/site/publication/. Bulk and single-cell eQTL data used in this study, including peripheral blood and retinal datasets, are accessible at https://yanglab.westlake.edu.cn/software/smr/#DataResource, with additional retinal single-cell RNA-seq data accessible upon request to the corresponding authors of Daniszewski et al. 45. Neuronal single-cell RNA-seq data from Jerber et al. 46 can be found on Zenodo (https://zenodo.org/record/3625024). Methylation profile datasets used in this study are accessible at https://yanglab.westlake.edu.cn/software/smr/#DataResource, and drug target data were obtained from DGIdb (https://www.dgidb.org/). Supplementary data for Figs. 1–3 are included in Supplementary Data 7–9.
Code availability
The code used in this study is available upon request to the corresponding authors. Scripts will be provided to the requester within one to five business days.
Competing interests
A.P.K. has acted as a paid consultant or lecturer to Abbvie, Aerie, Allergan, Google Health, Heidelberg Engineering, Novartis, Reichert, Santen and Thea. J.H.K. has received research funding from Pfizer, Inc. S.M., J.E.C. and A.W.H. are co-founders of and hold stock in Seonix Pty Ltd. The remaining authors report no other competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Stuart MacGregor, Puya Gharahkhani.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Santiago Diaz-Torres, Email: Santiago.DiazTorres@qimrberghofer.edu.au.
Puya Gharahkhani, Email: Puya.Gharahkhani@qimrberghofer.edu.au.
IGGC International Glaucoma Genetics Consortium:
Xikun Han, Andrew R. Hamel, Terri L. Young, Andrew J. Lotery, Eric Jorgenson, Hélène Choquet, Michael Hauser, Jessica N. Cooke Bailey, Toru Nakazawa, Yukihiro Shiga, Ayellet V. Segrè, Anthony P. Khawaja, Christopher J. Hammond, Pirro G. Hysi, Louis R. Pasquale, Masato Akiyama, Alex W. Hewitt, David A. Mackey, Jamie E. Craig, Janey L. Wiggs, Jue-Sheng Ong, Stuart MacGregor, and Puya Gharahkhani
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-54301-2.
References
- 1.Wiggs, J. L. & Pasquale, L. R. Genetics of glaucoma. Hum. Mol. Genet.26, R21–R27 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang, N., Wang, J., Li, Y. & Jiang, B. Prevalence of primary open angle glaucoma in the last 20 years: a meta-analysis and systematic review. Sci. Rep.11, 13762 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang, K., Gaitsch, H., Poon, H., Cox, N. J. & Rzhetsky, A. Classification of common human diseases derived from shared genetic and environmental determinants. Nat. Genet.49, 1319–1325 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonas, J. B. et al. Ocular hypertension: general characteristics and estimated cerebrospinal fluid pressure. The Beijing Eye Study 2011. PLoS ONE9, e100533 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thonginnetra, O. et al. Normal versus high tension glaucoma: a comparison of functional and structural defects. J. Glaucoma19, 151–157 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lešták, J., Pitrová, Š., Nutterová, E. & Bartošová, L. Normal tension vs high tension glaucoma: an—overview. Cesk. Slov. Oftalmol.75, 55–60 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Iester, M., De Feo, F. & Douglas, G. R. Visual field loss morphology in high- and normal-tension glaucoma. J. Ophthalmol.2012, 327326 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mullany, S. et al. Normal-tension glaucoma is associated with cognitive impairment. Br. J. Ophthalmol. 10.1136/bjophthalmol-2020-317461 (2021). [DOI] [PMC free article] [PubMed]
- 9.Jayaram, H. Intraocular pressure reduction in glaucoma: does every mmHg count? Taiwan J. Ophthalmol.10, 255–258 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mallick, J., Devi, L., Malik, P. K. & Mallick, J. Update on normal tension glaucoma. J. Ophthalmic Vis. Res.11, 204–208 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chua, J. et al. Prevalence, risk factors, and visual features of undiagnosed glaucoma: the Singapore Epidemiology of eye diseases study. JAMA Ophthalmol.133, 938–946 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Nucci, C. et al. Neuroprotective agents in the management of glaucoma. Eye32, 938–945 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gharahkhani, P. et al. Genome-wide meta-analysis identifies 127 open-angle glaucoma loci with consistent effect across ancestries. Nat. Commun.12, 1258 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campos, A. I. et al. Insights into the aetiology of snoring from observational and genetic investigations in the UK Biobank. Nat. Commun.11, 817 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barton, A. R., Sherman, M. A., Mukamel, R. E. & Loh, P.-R. Whole-exome imputation within UK Biobank powers rare coding variant association and fine-mapping analyses. Nat. Genet.53, 1260–1269 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christakoudi, S., Evangelou, E., Riboli, E. & Tsilidis, K. K. GWAS of allometric body-shape indices in UK Biobank identifies loci suggesting associations with morphogenesis, organogenesis, adrenal cell renewal and cancer. Sci. Rep.11, 10688 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ying, H. & Yue, B. Y. J. T. Cellular and molecular biology of optineurin. Int. Rev. Cell Mol. Biol.294, 223–258 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ritch, R. et al. TBK1 gene duplication and normal-tension glaucoma. JAMA Ophthalmol.132, 544–548 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fingert, J. H. et al. Transgenic TBK1 mice have features of normal tension glaucoma. Hum. Mol. Genet.26, 124–132 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, D. et al. Overexpression of BMP4 protects retinal ganglion cells in a mouse model of experimental glaucoma. Exp. Eye Res.210, 108728 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Igarashi, N. et al. Aqueous autotaxin and TGF-βs are promising diagnostic biomarkers for distinguishing open-angle glaucoma subtypes. Sci. Rep.11, 1408 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hachana, S. & Larrivée, B. TGF-β superfamily signaling in the eye: implications for ocular pathologies. Cells11, 2336 (2022). [DOI] [PMC free article] [PubMed]
- 23.Wang, J. et al. Targeting transforming growth factor-β signaling in primary open-angle glaucoma. J. Glaucoma26, 390–395 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Wordinger, R. J., Sharma, T. & Clark, A. F. The role of TGF-β2 and bone morphogenetic proteins in the trabecular meshwork and glaucoma. J. Ocul. Pharmacol. Ther.30, 154–162 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keel, S. et al. Prevalence of glaucoma in the Australian National Eye Health Survey. Br. J. Ophthalmol.103, 191–195 (2019). [DOI] [PubMed] [Google Scholar]
- 26.MacGregor, S. et al. Genome-wide association study of intraocular pressure uncovers new pathways to glaucoma. Nat. Genet.50, 1067–1071 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Song, X.-Y. et al. Atorvastatin reduces IOP in ocular hypertension in vivo and suppresses ECM in trabecular meshwork perhaps via FGD4. Int. J. Mol. Med. 49, 76 (2022). [DOI] [PMC free article] [PubMed]
- 28.Schmeer, C., Gámez, A., Tausch, S., Witte, O. W. & Isenmann, S. Statins modulate heat shock protein expression and enhance retinal ganglion cell survival after transient retinal ischemia/reperfusion in vivo. Investig. Ophthalmol. Vis. Sci.49, 4971–4981 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Han, X. et al. Automated AI labeling of optic nerve head enables insights into cross-ancestry glaucoma risk and genetic discovery in >280,000 images from UKB and CLSA. Am. J. Hum. Genet.108, 1204–1216 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raina, P. et al. Cohort profile: the Canadian Longitudinal Study on Aging (CLSA). Int. J. Epidemiol.48, 1752–1753j (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raina, P. S. et al. The Canadian longitudinal study on aging (CLSA). Can. J. Aging28, 221–229 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Sudlow, C. et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med.12, e1001779 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forgetta, V. et al. Cohort profile: genomic data for 26 622 individuals from the Canadian Longitudinal Study on Aging (CLSA). BMJ Open12, e059021 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mbatchou, J. et al. Computationally efficient whole-genome regression for quantitative and binary traits. Nat. Genet.53, 1097–1103 (2021). [DOI] [PubMed] [Google Scholar]
- 35.Willer, C. J., Li, Y. & Abecasis, G. R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics26, 2190–2191 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turley, P. et al. Multi-trait analysis of genome-wide association summary statistics using MTAG. Nat. Genet.50, 229–237 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Randall, J. C. et al. Sex-stratified genome-wide association studies including 270,000 individuals show sexual dimorphism in genetic loci for anthropometric traits. PLoS Genet.9, e1003500 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bulik-Sullivan, B. K. et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet.47, 291–295 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pickrell, J. K. et al. Detection and interpretation of shared genetic influences on 42 human traits. Nat. Genet.48, 709–717 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li, A. et al. mBAT-combo: a more powerful test to detect gene–trait associations from GWAS data. Am. J. Hum. Genet.110, 30–43 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang, J. et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat. Genet.44, 369–375 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu, Z. et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat. Genet.48, 481–487 (2016). [DOI] [PubMed]
- 43.Lloyd-Jones, L. R. et al. The genetic architecture of gene expression in peripheral blood. Am. J. Hum. Genet.100, 371 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ratnapriya, R. et al. Retinal transcriptome and eQTL analyses identify genes associated with age-related macular degeneration. Nat. Genet.51, 606–610 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daniszewski, M. et al. Retinal ganglion cell-specific genetic regulation in primary open-angle glaucoma. Cell Genom.2, 100142 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jerber, J. et al. Population-scale single-cell RNA-seq profiling across dopaminergic neuron differentiation. Nat. Genet.53, 304–312 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu, Y. et al. Joint analysis of GWAS and multi-omics QTL summary statistics reveals a large fraction of GWAS signals shared with molecular phenotypes. Cell Genom.3, 100344 (2023). [DOI] [PMC free article] [PubMed]
- 48.Wu, Y. et al. Integrative analysis of omics summary data reveals putative mechanisms underlying complex traits. Nat. Commun.9, 918 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freshour, S. L. et al. Integration of the Drug–Gene Interaction Database (DGIdb 4.0) with open crowdsource efforts. Nucleic Acids Res.49, D1144–D1151 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The GWAS summary data for the meta-analysis generated in this study is available on Zenodo [10.5281/zenodo.14010557]. Individual-level data from the UK Biobank were accessed under application number 25331, and CLSA data under application number 190225. Researchers may obtain access to these datasets by applying directly to each cohort: UK Biobank (https://www.ukbiobank.ac.uk/) and CLSA. GWAS data from the IGGC in European and Asian populations used in this study are available upon request to the corresponding authors of Gharahkhani et al. 13; the POAG GWAS data used in this study can be accessed via the GWAS Catalog (GCST90011766). FinnGen normotensive glaucoma GWAS data used in this study is accessible at https://r8.finngen.fi/pheno/H7_GLAUCOMA_NTG. The GWAS summary statistics for vertical cup-disc ratio (VCDR) GWAS used in this study are available at https://xikunhan.github.io/site/publication/. Bulk and single-cell eQTL data used in this study, including peripheral blood and retinal datasets, are accessible at https://yanglab.westlake.edu.cn/software/smr/#DataResource, with additional retinal single-cell RNA-seq data accessible upon request to the corresponding authors of Daniszewski et al. 45. Neuronal single-cell RNA-seq data from Jerber et al. 46 can be found on Zenodo (https://zenodo.org/record/3625024). Methylation profile datasets used in this study are accessible at https://yanglab.westlake.edu.cn/software/smr/#DataResource, and drug target data were obtained from DGIdb (https://www.dgidb.org/). Supplementary data for Figs. 1–3 are included in Supplementary Data 7–9.
The code used in this study is available upon request to the corresponding authors. Scripts will be provided to the requester within one to five business days.