Take Home Message
The aim of our study is to assess whether flexible ureteroscopy with a suction bendable ureteral access sheath is comparable to mini-percutaneous nephrolithotomy in terms of the stone-free rate and perioperative outcomes.
Keywords: Urolithiasis, Retrograde intrarenal surgery, Ureteral access sheath, Percutaneous nephrolithotomy, Flexible urteroscopy, Tip bandable suction ureteral access sheath
Abstract
Guidelines recommend percutaneous nephrolithotomy (PCNL) for larger renal stones, but advances in flexible ureteroscopy (f-URS) with a tip-bendable suction ureteral access sheath (S-UAS) have prompted further investigation. Our multicenter, international, randomized controlled trial is investigating whether f-URS with S-UAS is noninferior to mini-PCNL (mPCNL) for renal stones of 2–3 cm. The primary objective is the immediate stone-free rate (SFR). Secondary outcomes include the 3-mo SFR, complication rates, surgical time, hospital stay, auxiliary procedures, and improvements in quality of life. A total of 720 patients from 12 urological centers will be randomized to either f-URS with S-UAS or PCNL. Statistical analyses will include intention-to-treat and per-protocol approaches, with specific methods for normally and non-normally distributed data. Subgroup analyses will focus on stone location and lithotripter types. The significance threshold will be set at p < 0.05. The aim of this trial is to generate high-level evidence regarding the noninferiority of f-URS with S-UAS compared to mPCNL for medium-sized renal stones.
The trial is registered on ClinicalTrials.gov as NCT06526390.
1. Background
According to the European Association of Urology and American Urological Association guidelines, percutaneous nephrolithomy (PCNL) is the preferred treatment option for renal stones larger than 2 cm [1], [2]. In recent years, advances in flexible ureteroscopy (f-URS) technology, such as the emergence of smaller disposable flexible ureteroscopes and suction techniques, have increased its efficiency and expanded the indications [3], [4], [5].
The tip-bendable suction ureteral access sheath (S-UAS) represents an innovative advance in f-URS technology; the exceptional tip flexibility and bendability allow the device to passively bend at angles greater than 90° in tandem with the ureteroscope [6]. An S-UAS can be integrated with a vacuum suction system. Preliminary research showed that an S-UAS can substantially improve the stone-free rate (SFR) while also reducing the complication rate. Given the significant improvement in the effectiveness of f-URS with S-UAS, several studies have reported favorable outcomes in the management of renal stones ≥2 cm using f-URS. However, high-level evidence on whether f-URS with S-UAS can achieve outcomes comparable to PCNL for medium-sized stones (2–3 cm) is still lacking.
The objective of the current study is to compare clinical outcomes of f-URS with S-UAS and mini-PCNL (mPCNL) in the treatment of 2–3-cm renal stones.
2. Study objectives
The aim of this study is to determine whether f-URS with S-UAS is noninferior to mPCNL in terms of surgical efficiency for 2–3 cm renal stones. An initial pilot phase will assess the feasibility of recruitment and the appropriateness of the eligibility criteria and outcome measures. The following research question will be addressed: does f-URS with S-UAS result in noninferior SFRs in comparison to mPCNL? Secondary objectives are to compare complication rates, surgical time, and hospitalization time.
3. Study design and methods
3.1. Study overview
The study is a multicentre, international, prospective, parallel group, noninferiority, randomized, controlled trial.
3.2. Setting
The study will take place across 12 urological departments with significant experience in the management of urinary stones. The centers include eight in China, one in Malaysia, one in Russia, one in India, and one in Turkey. Each participating center routinely conducts more than 300 f-URS and 100 mPCNL procedures annually.
3.3. Study population
The study inclusion criteria are as follows:
-
(1)
Adults aged ≥18 yr;
-
(2)
American Society of Anesthesiology score of 1–3;
-
(3)
Renal stone of 2–3 cm in diameter as confirmed via noncontrast computed tomography (CT); and
-
(4)
Able to provide written informed consent and adhere to the requirements of the trial.
The exclusion criteria are as follows:
-
(1)
Patients with abnormalities of the urinary tract anatomy (such as a horseshoe kidney or ileal conduit);
-
(2)
Patients with an untreated urinary tract infection;
-
(3)
Patients with health conditions or other factors that serve as absolute contraindications to f-URS or mPCNL; and
-
(4)
Patients unable to understand or complete the trial documentation.
3.4. Identification and enrolment of potential participants
Local procedures at the participating hospitals may differ; hence, the approach to patients and the consent process will be tailored to fit both site-specific practices and patient needs. Clinicians or designated personnel will assess patients presenting with suspected renal stones as part of standard practice. A log will be kept of all patients assessed to document reasons for their non-inclusion in the study, such as ineligibility or refusal to participate, to inform the CONSORT diagram. Brief details of potentially eligible patients will be recorded in the screening logs at each site to aid in monitoring participant inclusion.
Once renal stones are confirmed by CT scan, eligible patients, based on the inclusion and exclusion criteria, will receive a patient information leaflet. This leaflet will explain the benefits and known drawbacks of all aspects of the trial, which investigates the use of either f-URS or mini-PCNL as the treatment for renal stones. Patients will have the opportunity to discuss the study with the local clinical team and may choose to participate during a clinic appointment or while hospitalized for their initial stone episode.
Signed informed consent forms will be obtained from participants at all centers. Participants who are unable to give informed consent (e.g., due to incapacity) will not be eligible for the study. Permission will be sought from participants to inform their general practitioner of their involvement in the trial. After providing consent and completing a baseline questionnaire, participants will be randomly assigned to one of the two treatment groups.
3.5. Randomization and allocation
Randomization will be performed using a stratified method according to the participating centers. Each of the 12 centers will enroll 60 participants. Participants at each center will be randomized to either f-URS or mPCNL in a 1:1 ratio. Randomization sequence generation will be performed electronically before patient participation. Random sequence allocation and concealment will be implemented using consecutively numbered, sealed envelopes. When the patient has signed the informed consent form and the decision for surgery has been made, the envelope will be opened to determine the surgical method.
3.6. Intervention
Two interventions will be evaluated: (1) f-URS with S-UAS and (2) mPCNL.
3.7. Surgical protocol
A standardized operating methodology, approved by the principal investigator at each center, will be established to ensure uniformity. Monthly protocol monitoring visits will be conducted at all participating centers to ensure adherence to the standardized procedures.
A noncontrast CT scan with a 2-mm slice thickness will be routinely performed to determine the stone location and size, including the diameter, area, and volume. The stone burden and density will be measured consistently using the same software across all centers. All patients will receive standard perioperative antibiotic prophylaxis, with a single dose of cefuroxime 200 mg or levofloxacin 500 mg (for those allergic to cefuroxime) administered 0.5 h before the procedure in accordance with the standard practice at each center. Patients with a positive preoperative urine culture will receive appropriate antibiotics according to culture sensitivity results for 4–7 d before their procedure.
3.7.1. Endoscopic procedure: f-URS
The procedure will be performed under general anesthesia with the patient in the lithotomy position. A 5-Fr open-ended ureteral catheter will be inserted into the ureter, followed by retrograde pyelography to evaluate the upper urinary tract. A 0.035/0.038-inch guidewire will then be advanced into the renal pelvis. Depending on the case, either a 12/14-Fr or 11/13-Fr S-UAS will be used. If the UAS cannot be placed because of a narrow ureter, a smaller 10/12-Fr UAS will be attempted. If the 10/12-Fr UAS also fails, a double-J stent will be inserted and the procedure will be halted. A second f-URS session will be scheduled 2 wk after double-J stent placement.
f-URS will be performed using digital flexible ureteroscopes of two sizes: 8.5 Fr and 7.5 Fr. Ureteroscope selection will be based on the UAS size: an 8.5-Fr scope for a 12/14-Fr UAS, and a 7.5-Fr scope for an 11/13-Fr or 10/12-Fr UAS.
The stone will be fragmented using either holmium laser (Ho:YAG) or thulium fiber laser (TFL) with a 200-μm laser fiber and an energy setting of <30 W. An irrigation pressure pump will be used, with the flow rate set at 50–100 ml/min and suction pressure controlled at 80–120 mmHg. At the end of the procedure, both the UAS and ureteroscope will be removed.
A 6-Fr indwelling double-J stent will be placed postoperatively and retained for 2–4 wk, depending on the condition of the ureter. Routine placement of a postoperative Foley catheter will not be performed.
3.7.2. mPCNL
With the patients under general anesthesia, a 5-Fr ureteral catheter will be inserted into the target ureter using a ureteroscope, and the bladder will be drained with a 16-Fr Foley catheter. The patient will then be turned to the prone position. Percutaneous access will be achieved using an 18-gauge coaxial needle to puncture the desired calyx under fluoroscopic or ultrasound guidance. Tract dilatation will be accomplished using fascial dilators up to 18 Fr. When multiple nephrostomy tracts are necessary to remove the stones, the same technique will be applied for each tract. Fragmentation of the stone burden will be accomplished using either a pneumatic lithotripter, Ho:YAG laser, or TFL. At the end of the procedure, a 6-Fr double-J ureteral stent will be left in place. The decision to leave a silastic nephrostomy tube will be left to the clinical discretion of each operating surgeon.
3.8. Follow-up and data collection
A low-dose, noncontrast CT scan with a 2-mm slice thickness will be conducted within 72 h after the procedure to evaluate the immediate SFR. SFR is defined as the absence of residual stones visible under endoscopy and either no fragments or fragments ≤2 mm in the kidney. Routine blood tests and serum procalcitonin levels will be assessed within 2 h after surgery to monitor for infection. Any decrease in hemoglobin at 24 h after surgery in comparison to the preoperative level will be recorded. Patients with a postoperative hemoglobin level <80 g/l will receive a whole-blood transfusion.
For the f-URS group, patients without significant postoperative discomfort will be discharged the day after surgery. In the mPCNL group, if a nephrostomy tube was placed, it will be removed 1–3 d after the drainage becomes grossly clear, followed by patient discharge. The double-J stent will be removed 2 wk after surgery. Quality of life (QoL) scores will be assessed both preoperatively and 1 mo postoperatively. Stone composition will be analyzed using the same infrared spectrometer and methodology at all centers. Low-dose CT scans will be performed at 3-mo follow-up to determine the final SFR.
Patient characteristics and clinical outcomes will be carefully recorded using pre-established case report forms (CRFs). Stone size will be defined as the largest diameter for a single stone or the sum of the largest diameters for multiple stones. Stone size and Hounsfield units (HU) will be measured consistently using the same software across all centers. Hydronephrosis will be classified according to the 2016 Onen grading system. Operative time will be measured from insertion of the endoscope into the urethra to completion of stent placement for the f-URS group, and from percutaneous puncture to completion of nephrostomy tube placement for the mPCNL group. Hospital stay will be rounded to the nearest whole day, calculated from the day of surgery to the day of discharge.
The research team at the recruiting site will complete four CRFs: one at randomization, one after the intervention, and two at 1-mo and 3-mo follow-up, including details of any additional interventions and complications. Table 1 shows the schedule for outcome assessment and data collection.
Table 1.
Schedule for data collection and outcome assessment
| Outcome measure | Timing |
||||
|---|---|---|---|---|---|
| Recruitment | Intervention |
Follow-up |
|||
| During surgery |
Before discharge |
1 mo | 3 mo | ||
| Baseline assessment Health status Stone location and size ASA classification Preoperative laboratory tests Preoperative imaging |
✓ | ||||
| Quality of life score | ✓ | ✓ | |||
| Operative characteristics Operative time Irrigation fluid volume Degree of ureteral wall injury Operative complications |
✓ | ||||
| Postoperative laboratory tests | ✓ | ||||
| Duration of hospital stay | ✓ | ||||
| Complications | ✓ | ✓ | ✓ | ✓ | |
| Additional interventions received | ✓ | ✓ | ✓ | ||
| Immediate stone-free status | ✓ | ||||
| Final stone-free status | ✓ | ||||
ASA = American Society of Anesthesiologists.
3.9. Outcome measures
3.9.1. Primary outcome
For the immediate SFR, stone-free status is defined as no residual stone visualized under endoscopy and no residual stone fragments or fragments <2 mm on a low-dose CT scan with a 2-mm slice thickness within 72 h after surgery.
3.9.2. Secondary outcomes
Secondary outcomes are as follows:
-
(1)
Final SFR: SFR at 3-mo follow-up according to CT scans.
-
(2)
Operative time: for the mPCNL group, operative time is defined from retrograde placement of the ureteral catheter to completion of nephrostomy. For the f-URS group, the operative time is defined from insertion of the endoscope into the urethra to completion of stent placement.
-
(3)
Duration of hospital stay: time rounded to the nearest whole day and calculated from the day of surgery to the day of discharge.
-
(4)
Further interventions received up to 3 mo after randomization.
-
(5)
Complications occurring up to 3 mo after randomization will be evaluated using the Clavien-Dindo grading system [7].
-
(6)
Improvement in QoL score: QoL will be assessed preoperatively and at 1 mo postoperatively using the Wisconsin Stone QoL questionnaire [8].
3.10. Blinding
Blinding of patients and surgeons to the assigned trial arm is not feasible because of the inherent differences in the interventions. However, radiologists who evaluate the postoperative imaging (CT scans) will be blinded to the specific intervention received. The postoperative clinical assessment will be performed by investigators who are blinded to the surgical procedures and were not involved in the surgeries. We will perform blinded statistical analyses in which the individual performing the analyses is unaware of the group to which each patient belongs. The code for group assignment will remain concealed until the analyses and interpretation have been completed.
3.11. Subject withdrawal
Participants will continue in the trial unless they opt to withdraw their consent or are unable to proceed because of clinical reasons. If a participant withdraws consent, efforts will be made to obtain permission to persist in collecting outcome data from their health care records. Any other changes in participant status, aside from formal withdrawal of consent, will result in continued follow-up for all study outcomes where feasible.
3.12. Sample size
The initial sample-size calculations are based on a noninferiority design for the trial. Preliminary data indicate that the SFR immediately following treatment is expected to be ∼85% (P1) for the mPCNL group and ∼80% (P2) for the f-URS group when treating 2–3-cm renal stones. An acceptable margin of inferiority has been set at 8%, meaning P2 −P1 > −8%. Using simulations conducted in STATA (StataCorp, College Station, TX, USA) with 80% power and an α level of 2.5%, the sample size required is 313 participants per group (totaling 626). To accommodate potential losses to follow-up and withdrawals, this number was adjusted to 360 participants per group (720 in total).
3.13. Statistical analysis
Data will be compared across different groups using the intention-to-treat (ITT) principle. This approach ensures that all patients are included in the analysis according to the group they were originally assigned to, regardless of follow-up status or dropout. Missing data will be addressed using multiple imputation within the ITT analysis. A sensitivity analysis will be conducted to compare the ITT results with a per-protocol data set that includes only those patients who adhered to the study protocol.
Statistical analyses will be performed using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). Primary and secondary outcomes will be evaluated as follows. Continuous variables with a normal distribution will be presented as the mean ± standard deviation, while non-normally distributed data will be reported as the median with interquartile range. Between-group comparisons of normally distributed continuous variables will use an independent-sample t test, while non-normally distributed data will be compared using the Mann-Whitney U test. Count data will be expressed as case numbers (rates) and analyzed using a χ2 test or Fisher’s exact test. For multivariable analyses, multiple linear or logistic regression models will be used, depending on whether the dependent variables are continuous or categorical. Results will be presented as estimates with 95% confidence intervals. Subgroup analyses will investigate potential effect modification by stone location and the energy/type of lithotripter used for stone fragmentation in treatment-by-subgroup interaction tests. A p value of <0.05 will be considered statistically significant.
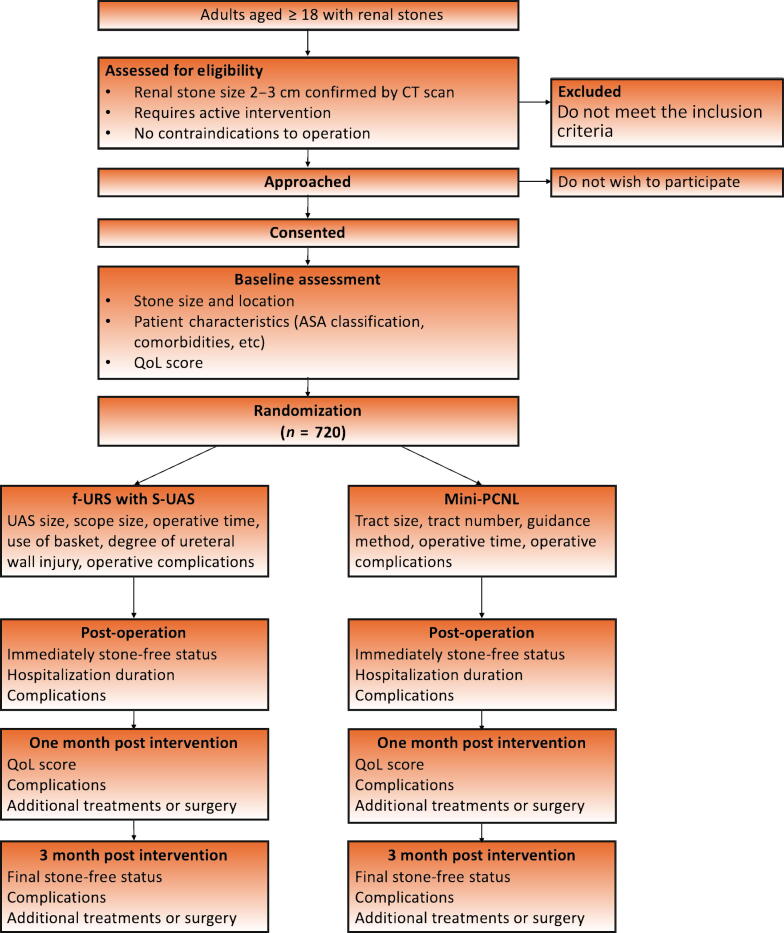
3.14. Study flow chart
Figure 1 summarizes the patient enrollment process for the study.
Fig. 1.
Flowchart of the study inclusion process. CT = computed tomography; QoL = quality of life.
4. Quality control
Participating centers have sufficient numbers of urolithiasis cases to ensure that the target number of patients is enrolled. Each center routinely conducts more than 300 f-URS and 100 mPCNL procedures annually. A study period of 1 yr is sufficient to enroll 720 eligible patients. We will strictly adhere to the inclusion, exclusion, and withdrawal criteria. Preoperative patient evaluations will be completed by urologists with extensive experience in urolithiasis. This trial draws on quality control expertise from experts in urology, endourological surgery, statistics, and research departments. The trial will be conducted by senior urologists, intermediate urologists, junior urology trainees, and postgraduate students working together to ensure quality at all levels. A standardized operating methodology, approved by the principal investigator at each center, has been implemented to ensure consistency. Monthly protocol monitoring visits will be conducted at all participating centers to verify adherence to the established procedures. Each center will have one designated, experienced surgeon (with at least 100 f-URS and mPCNL procedures each per year) performing the procedures.
Any adverse events that arise during the trial will be documented on the CRF and reported to the principal investigator. Each adverse event will be evaluated based on its type (expected vs unexpected), severity (serious vs non-serious), and its relationship to the intervention (relevant vs irrelevant). Serious and unexpected adverse events will be reported to the ethics committee. The principal investigator will carry out regular cumulative reviews of all adverse events and will organize investigator meetings as needed.
5. Publication plan
We expect the study to determine whether f-URS with S-UAS achieves a noninferior SFR in comparison to mPCNL. The results will be published in a peer-reviewed international journal. In addition, information about the study and its findings will be disseminated via presentations at various urological meetings and conventions.
6. Discussion
Endourology has witnessed significant advances in recent years, particularly with the introduction of the S-UAS [6]. A remarkably high SFR of 97.2% with this innovative device has been demonstrated, suggesting a significant improvement in the management of renal stones [9]. The S-UAS enhances maneuverability and access within the renal collecting system, potentially mitigating some of the limitations of traditional f-URS methods.
Previous studies comparing mPCNL and f-URS have had varied outcomes, particularly concerning stone location. For lower-pole stones, a clear advantage of mPCNL has been demonstrated, with significantly higher SFRs, as difficult retrograde access and reduced maneuverability of the ureteroscope in the lower pole compromise the success of f-URS [10].
A systematic review and meta-analysis of 590 patients with stones >2 cm revealed similar SFRs for mPCNL and f-URS. However, f-URS was associated with a shorter hospital stay and a lower rate of bleeding complications [11]. Bai et al [12] found comparable SFRs at 3-mo follow-up for stones larger than 2 cm (82.1% vs 88.3%; p = 0.346). While f-URS was associated with a longer operative time, it resulted in a significantly shorter postoperative hospital stay [12].
In one study, the overall complication rate was slightly higher for mPCNL (15.8% vs 9.3% for f-URS) [10] although the difference was not statistically significant. Comparison of specific complications revealed a greater average decrease in hemoglobin in the mPCNL group. Despite the higher complication rate for mPCNL, the differences were not statistically significant, indicating that both procedures have acceptable safety profiles.
A study by Zhu et al [6] comparing S-UAS with traditional UAS for f-URS highlighted several S-UAS advantages. The S-UAS group had a significantly higher immediate SFR than the control group, and a higher SFR at 3 mo after the intervention. The S-UAS group also had lower rates of postoperative fever and use of stone baskets, and a greater improvement in QoL. There were no significant differences between the groups in operative time, hospital stay, or the rate of repeat f-URS.
Given the promising outcomes associated with S-UAS and the established efficacy of mPCNL, the aim of our study is to directly compare these two techniques for the treatment of 2–3-cm renal stones. The primary objective is to determine whether f-URS using S-UAS is noninferior to mPCNL in terms of SFR, with secondary outcomes including complication rates, hospital stay, operative time, and QoL after surgery.
By conducting an international, multicentre, randomized, parallel-group, noninferiority study, we aim to provide robust evidence to guide clinical decision-making in the management of medium-sized renal stones. This study will contribute to the growing body of literature in endourology and potentially establish the f-URS with S-UAS as a viable alternative to mPCNL, offering patients a less invasive option with comparable efficacy and safety.
7. Planned progress
Inclusion of patients will start in August 2024 and continue up to July 2025. Follow-up will be performed continuously according to the study protocol. We expect the final 3-mo follow-up to be completed by October 2025. After the last follow-up visit, we will perform statistical analyses of the data and results, and will publish the first study results as soon as possible, with a target of December 2025.
Author contributions: Guohua Zeng had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Zeng, Zhu, Chai.
Acquisition of data: Zhu, Chai, Gökce, Gadzhiev, Kalathia, Jiang, Duan, Cao, Wu, Song, Bai, Li, Liu, Zeng.
Analysis and interpretation of data: Ma.
Drafting of the manuscript: Zhu, Chai.
Critical revision of the manuscript for important intellectual content: Zeng.
Statistical analysis: Ma.
Obtaining funding: Zeng.
Administrative, technical, or material support: Chai, Ma, Gökce, Gadzhiev, Kalathia.
Supervision: Zeng.
Other: None.
Financial disclosures: Guohua Zeng certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This work was financed by grants from the National Natural Science Foundation of China (grants 82070721 and 82270822) and the Fund for Enhancing Scientific Research of Guangzhou Medical University (grant GMUCR2024-01006). The sponsors have no direct role in the study.
Associate Editor: Silvia Proietti
Contributor Information
Wei Zhu, Email: doczw1989@126.com.
Chu Ann Chai, Email: chaichuann@yahoo.com.
Guohua Zeng, Email: gzgyzgh@vip.sina.com.
References
- 1.Assimos D, Krambeck A, Miller NL, et al. Surgical management of stones: American Urological Association/Endourological Society guideline. Linthicum, MD: American Urological Association; 2016. https://www.auanet.org/guidelines/guidelines/kidney-stones-surgical-management-guideline.
- 2.Skolarikos A., Jung H., Neisius A., et al. The Netherlands; European Association of Urology; Arnhem: 2024. EAU guidelines on urolithiasis. https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-Guidelines-on-Urolithiasis-2024.pdf. [Google Scholar]
- 3.Chen Y., Zheng L., Lin L., et al. A novel flexible vacuum-assisted ureteric access sheath in retrograde intrarenal surgery. BJU Int. 2022;130:586–588. doi: 10.1111/bju.15873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alenezi H., Denstedt J.D. Flexible ureteroscopy: technological advancements, current indications and outcomes in the treatment of urolithiasis. Asian J Urol. 2015;2:133–141. doi: 10.1016/j.ajur.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taguchi K., Usawachintachit M., Tzou D.T., et al. Micro-costing analysis demonstrates comparable costs for LithoVue compared to reusable flexible fiberoptic ureteroscopes. J Endourol. 2018;32:267–273. doi: 10.1089/end.2017.0523. [DOI] [PubMed] [Google Scholar]
- 6.Zhu W., Liu S., Cao J., et al. Tip bendable suction ureteral access sheath versus traditional sheath in retrograde intrarenal stone surgery: an international multicentre, randomized, parallel group, superiority study. eClinicalMedicine. 2024;74 doi: 10.1016/j.eclinm.2024.102724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitropoulos D., Artibani W., Biyani C.S., Bjerggaard Jensen J., Rouprêt M., Truss M. Validation of the Clavien-Dindo grading system in urology by the European Association of Urology Guidelines Ad Hoc Panel. Eur Urol Focus. 2018;4:608–613. doi: 10.1016/j.euf.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Penniston K.L., Antonelli J.A., Viprakasit D.P., et al. Validation and reliability of the Wisconsin Stone Quality of Life questionnaire. J Urol. 2017;197:1280–1288. doi: 10.1016/j.juro.2016.11.097. [DOI] [PubMed] [Google Scholar]
- 9.Gauhar V, Traxer O, Castellani D, et al. Could use of a flexible and navigable suction ureteral access sheath be a potential game-changer in retrograde intrarenal surgery? Outcomes at 30 days from a large, prospective, multicenter, real-world study by the European Association of Urology Urolithiasis Section. Eur Urol Focus. In press. 10.1016/j.euf.2024.05.010. [DOI] [PubMed]
- 10.Mahmood S.N., Ahmed C., Tawfeeq, et al. Evaluation of mini-PCNL and RIRS for renal stones 1–2 cm in an economically challenged setting: a prospective cohort study. Ann Med Surg. 2022;81 doi: 10.1016/j.amsu.2022.104235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng C., Xiong B., Wang H., et al. Retrograde intrarenal surgery versus percutaneous nephrolithotomy for treatment of renal stones >2 cm: a meta-analysis. Urol Int. 2014;93:417–424. doi: 10.1159/000363509. [DOI] [PubMed] [Google Scholar]
- 12.Bai Y., Wang X., Yang Y., Han P., Wang J. Percutaneous nephrolithotomy versus retrograde intrarenal surgery for the treatment of kidney stones up to 2 cm in patients with solitary kidney: a single centre experience. BMC Urol. 2017;17:9. doi: 10.1186/s12894-017-0200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]