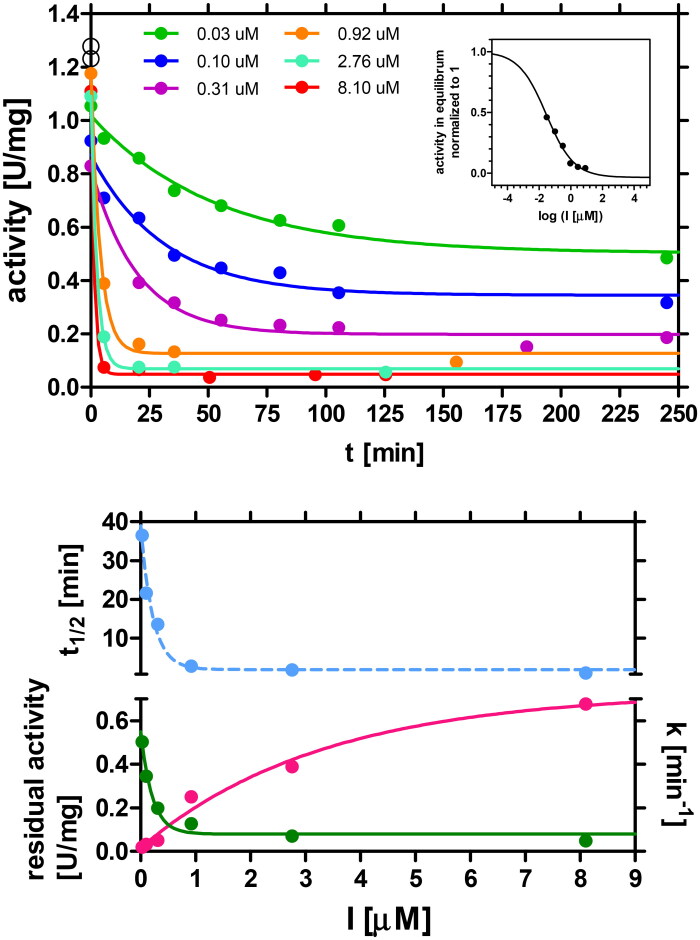
Figure 3.
Upper panel: Time dependence of inactivation of H. pylori AdSS by PLP present in various concentrations. Enzyme (22 nM) was incubated at 25 °C in 20 mM Hepes buffer at pH 7.7, with various concentrations of PLP: 0.03 µM (green), 0.1 µM (blue), 0.3 (violet), 0.92 µM (orange), 2.76 µM (aquamarine), 8.1 µM (red). Activity at time 0 and no PLP present is shown by the black open circles. One-phase exponential decay (Equation (2)) was fitted to each of the traces to get for each of the PLP concentrations the rate constant, k, of the inactivation process and the residual enzyme activity when equilibrium is reached, Aeq, and calculate half-time of the inactivation process t1/2. The inset shows activity in equilibrium vs. inhibitor concentration, with the four-parameter dose-response curve (Equation (1)) fitted, which yields IC50eq = 0.028 µM (with asymmetrical error range from 0.011 to 0.068 µM). Lower panel: Dependence of the rate constant, k, residual enzyme activity, Aeq, and half-time of the inactivation process, t1/2, on the inhibitor, PLP, concentration [I]. At saturation with the inhibitor these parameters are: k = 0.73 ± 0.09 min−1 (magenta); activity Aeq = 0.08 ± 0.03 U/mg (green) and t1/2 = 0.95 ± 0.11 min (blue).