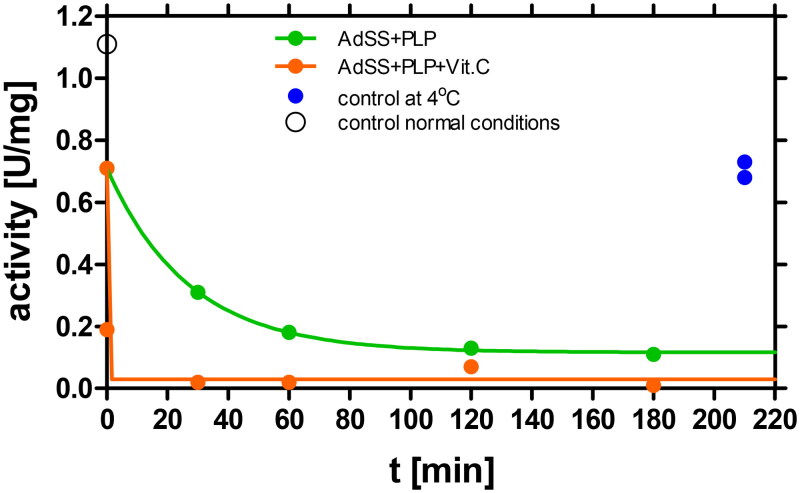
Figure 4.
Reduction by vitamin C of the Schiff base formed between AdSS and PLP. The enzyme, 4.15 µM, was incubated at 25 °C in 20 mM Hepes buffer at pH 7.7 with 100 µM PLP in the absence (green) and in the presence (orange) of 1 mM vitamin C. Enzyme activity assayed in the standard conditions, observed at the start of the experiment prior to addition of PLP and vitamin C, and at the end of the experiment for the sample without PLP and vitamin C kept in ice, is marked by a black open circle and a blue dots, respectively. In the presence of 1 mM of vitamin C the rate constant k increases from k = 0.037 ± 0.002 min−1 to k = 14.47 ± 1.86 min−1, while the residual enzyme activity Aeq, drops from Aeq = 0.12 ± 0.01 U/mg to practically zero, Aeq = 0.03 ± 0.02 U/mg.