Abstract
Millions of new cases of cancer are diagnosed worldwide each year, making it a serious public health concern. Developments in customized therapy and early detection have significantly enhanced treatment for and results from cancer. Therefore, it is important to investigate new molecular biomarkers. In this study, we created an efficient transient receptor potential melastatin (TRPM) family members-related TRPM-Score for 17 solid tumors. CCNE1, produced from TRPM-Score, was found to be an exceptional biomarker through several sophisticated machine learning and deep learning computational techniques. TRPM-Score and CCNE1 immunotherapeutic prediction, immunological characteristics, and predictive value were thoroughly assessed. In most cancer types, CCNE1 was a substantially dangerous marker. Additional in vitro tests validated CCNE1’s immunomodulatory properties, demonstrating that silencing impeded macrophage movement and decreased PD-L1 expression. Additionally, CCNE1 may accurately predict responses to cancer immunotherapy. These findings indicate that the TRPM family—particularly CCNE1, which is associated with TRPM—is a significant player in the pan-cancer domain and can be utilized as a therapeutic target and prognostic biomarkers, especially in immuno-oncology. The thorough characterization of the TRPM family and the discovery of CCNE1 as a crucial downstream effector mark important developments in our comprehension of pan-cancer biology.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12943-024-02169-7.
Keywords: CCNE1, Cancer immunotherapy, TRPM family, Macrophage, Tumor microenvironment
Introduction
Cancer remains a significant global public health challenge, with millions of new cases diagnosed annually. Advances in early detection and personalized medicine have markedly improved cancer treatment and patient outcomes. Consequently, identifying and exploring novel molecular biomarkers is of great importance. Transient receptor potential (TRP) channels represent a family of ion channels that are integral to various physiological and pathological processes, including sensory function, calcium and ion homeostasis, cell proliferation, neurological and cardiovascular function, as well as metabolic, endocrine regulation, and cancer progression [1]. Certain TRP channel subtypes, such as TRPV1, TRPV2, TRPC1, TRPC6, and TRPM8, exhibit differential expression patterns in cancer cells compared to normal tissues [2]. These alterations in TRP channel expression and activity can contribute to the hallmarks of cancer, such as sustained proliferation, evasion of apoptosis, increased angiogenesis, and enhanced metastatic potential [3]. The TRPM (Melastatin) family of channels belongs to the subclass of TRP channels with different permeabilities to Mg2 + and Ca2+ [4]. TRPM channels are typically tetrameric, consisting of four identical or similar subunits. Each subunit has six transmembrane segments (S1-S6). The S5 and S6 segments form the channel pore, while S1-S4 are involved in sensing stimuli. The N- and C-termini are large and contain various regulatory motifs, including ankyrin repeats and other functional domains that modulate channel activity. TRPM channels, while not as extensively studied as other ion channel families (e.g., TRPV or TRPC), offer new avenues for researching health and disease. Different TRPM family members have been implicated with oncogenic [5] and immunogenic [6] roles in different types of cancer. Preclinical research has identified TRPM family members as possible novel therapeutic targets in human disease [7]. Specifically, targeting TRPM2 is an effective tactic for avoiding and overcoming osimertinib-induced acquired resistance in lung cancer [8].
In this study, we developed a TRPM-Score based on the expression profiles of TRPM family members across 17 solid cancers. Our analysis revealed CCNE1 as a significant biomarker, identified by applying advanced computational approaches, including machine learning and deep learning techniques. The TRPM-Score and CCNE1 were further evaluated for their predictive value, immunological characteristics, and potential to guide immunotherapy response, providing a comprehensive assessment of their role in cancer prognosis and treatment.
Results
Comprehensive characterization of the TRPM family
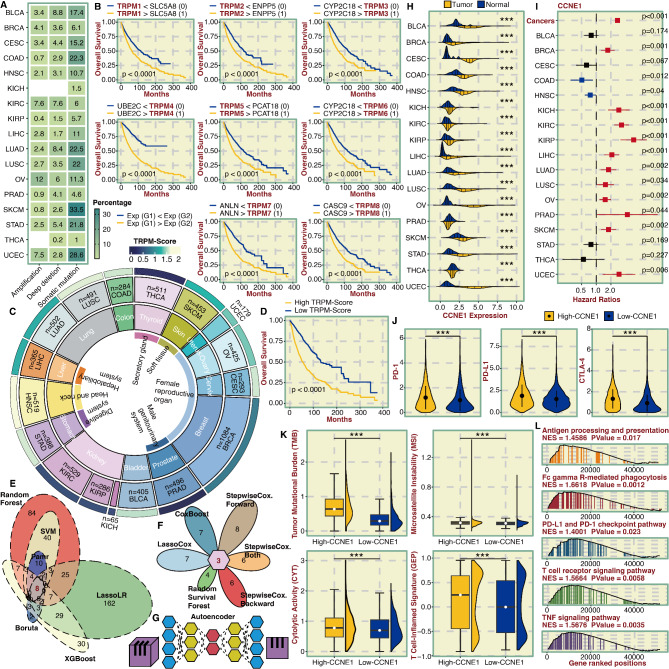
The mutation frequency of TRPM family members across pan-cancer was revealed, and somatic mutation of TRPM members frequently occurred in SKCM and UCEC (Fig. 1A). As shown in Figure S1A, the total mutation rate of the TRPM family members ranged from 10 to 29%, with TRPM6 showing the greatest degree of mutation (29%), predominantly consisting of missense mutations. Besides, the distribution of mutation frequencies (Figure S1B), amplification of SCNA frequencies (Figure S1C), and depletion of SCNA frequencies (Figure S1D) are presented, respectively. TRPM family members were generally more expressed in tumor tissues compared with normal tissues (Figure S1E). In addition, TRPM family members were found to be prognostic markers in solid cancer types (Figure S1F).
Fig. 1.
Identification and predictive potential of TRPM-Score and CCNE1. A. Heatmap showing mutation frequency (amplification, deep deletion, and somatic mutation) of TRPM family members in pan-cancer. B. Survival curves of the eight most potent gene pairs related to TRPM family members. Each gene pair was assigned 0 and 1 based on their relative expression values. C. The distribution of TRPM-Score in pan-cancer. D. Survival curves of the TRPM-Score-based groups. E. Venn plot showing the intersected genes from six machine learning algorithms for classification for dimension reduction. F. Petal chart showing the intersected genes from six machine learning algorithms for survival for further dimension reduction. G. Schematic diagram for deep learning, Autoencoder. H. Violin plot showing the expression difference of CCNE1 in tumor and normal tissues in pan-cancer. I. Univariate Cox regression analysis of CCNE1 in pan-cancer. J. Violin plot showing the expression difference of PD-1, PD-L1, and CTLA-4 in CCNE1-based groups in pan-cancer. K. Violin plot showing TMB, CYT, MSI, and GEP levels in CCNE1-based groups in pan-cancer. L. GSEA on CCNE1 in pan-cancer
Development process and annotation of the TRPM-Score
The flow diagram for the development of the TRPM-Score is shown in Figure S2. TRPM family members were independently paired with the prognostically notable genes to determine the most potent gene pairs based on the predictive ability (Fig. 1B). Eight gene pairs with discriminative ability in survival outcomes were used as the input for a LassoCox-based model to calculate a TRPM-Score (Figure S3A). The distribution of the TRPM-Score in pan-cancer is shown in Fig. 1C. Patients were stratified into high and low TRPM-Score groups based on the median TRPM-Score, with those in the high TRPM-Score group exhibiting significantly poorer survival outcomes (Fig. 1D). TRPM-Score was a significant hazardous marker in most cancer types (Figure S3B and S4A). Next, we explored the immunological features related to the TRPM-Score. In addition, the TRPM-Score was significantly associated with the antigen presentation process, chemokine & cytokine signaling, and TNF in pan-cancer (Figure S3C). Classical immune checkpoint molecules, PD-1, PD-L1, PD-L2, and CTLA-4, were significantly more expressed in the high TRPM-Score group (Figure S3D). Additionally, key immunotherapy biomarkers, TMB, MSI, CYT, and GEP, were significantly higher in the high TRPM-Score group (Figure S3E). Given the success of immunotherapy, we focused specifically on the potential of TRPM-Score to guide personalized immunotherapy. Notably, SKCM patients with elevated TRPM-Scores exhibit significantly lower Immune Scores and increased Tumor Purity (Figure S5A). Furthermore, elevated TRPM-Scores are associated with poorer survival across six independent SKCM cohorts (Figure S5B) and a reduced likelihood of response to ICB therapy (Figure S5C). Similarly, SKCM patients with high TRPM-Scores in ICB cohorts exhibit worse survival outcomes (Figure S5D). In NSCLC, STAD, and GBM, high TRPM-Scores correlate with significantly lower Immune Scores (Figure S5E, S5G, and S5I) and decreased responsiveness to ICB therapy (Figure S5F, S5H, and S5J). In contrast, KIRC and LIHC patients with high TRPM-Scores show markedly higher Immune Scores (Figures S5K and S5M) and an increased probability of responding to ICB therapy (Figures S5L and S5N).
Identification and predictive potential of TRPM family-related CCNE1
DEGs between high and low TRPM-Score groups were identified, and their significance was evaluated using a three-step advanced computational approach. Firstly, six machine learning algorithms for classification, SVM, Pamr, Boruta, XGBoost, LassoLR, and Random Forest, were applied to determine the most potent DEGs (Fig. 1E). Secondly, six machine learning algorithms for survival, CoxBoost, StepwiseCox Forward, StepwiseCox Both, StepwiseCox Backward, Random Survival Forest, and LassoCox, were applied to determine prognostically notable DEGs (Fig. 1F). Thirdly, a deep learning algorithm, Autoencoder, was used to determine the most valuable DEG, CCNE1 (Fig. 1G). CCNE1 was significantly more expressed in tumor tissues than in normal tissues in most cancer types (Fig. 1H and S4B). Besides, CCNE1 emerged as a significant hazardous marker in most cancer types (Fig. 1I and S4C). Classical immune checkpoint molecules, PD-1, PD-L1, and CTLA-4, were more highly expressed in the high CCNE1 group (Fig. 1J). Additionally, immunotherapy biomarkers, TMB, MSI, CYT, and GEP were significantly elevated in the high CCNE1 group (Fig. 1K). Next, we delved into the tumorigenic and immunogenic characteristics of CCNE1. CCNE1 played a key role in antigen presentation, PD-1/PD-L1 signaling, and TCR signaling in pan-cancer, as shown in Fig. 1L. Additionally, it actively regulated T cell differentiation, cytokine production, T cell-mediated cytotoxicity, and response to IFNγ, as illustrated in Figure S6A. Furthermore, CCNE1 is closely related to pathways such as apoptosis, cell cycle, lymphocyte-mediated immunity, and inflammatory mediator regulation of TRP channels, detailed in Figure S6B. We also paid special attention to the relevance of CCNE1 and immunotherapy. SKCM patients with high CCNE1 expression have significantly lower Immune Scores and higher Tumor Purity (Figure S7A). CCNE1 predicts worse survival in six independent SKCM cohorts (Figure S7B). Moreover, SKCM patients with high CCNE1 expression are less likely to respond to ICB therapy (Figure S7C). Besides, CCNE1 predicts worse survival in SKCM ICB cohorts (Figure S7D). Similarly, the Immune Scores are significantly lower in NSCLC (Figure S7E), STAD (Figure S7G), and GBM (Figure S7I) patients with high CCNE1 expression. NSCLC (Figure S7F), STAD (Figure S7H), and GBM (Figure S7J) patients with high CCNE1 expression are less likely to respond to ICB therapy. In contrast, Immune Scores are markedly higher in KIRC (Figure S7K) and LIHC (Figure S7M) patients with high CCNE1 expression, and KIRC (Figure S7L) and LIHC (Figure S7N) patients show a greater likelihood of responding to ICB therapy.
In vitro validation on CCNE1
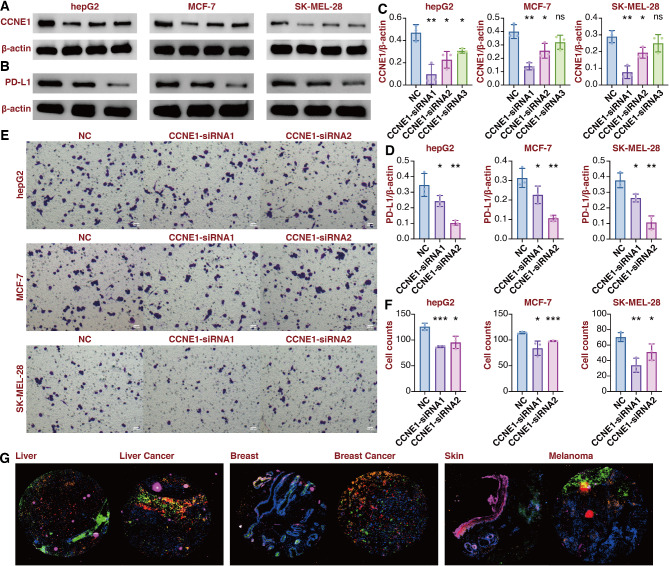
Given the predictive value of CCNE1 in cancer, in vitro validation was performed to corroborate our findings. The immunogenic roles of CCNE1 were explored in three commonly seen cancer types: LIHC, BRCA, and SKCM. Three siRNA sequences were used to silence the expression of CCNE1 in hepG2, MCF-7, and SK-MEL-28 cells (Fig. 2A and C). The two most powerful siRNA sequences were picked for the follow-up experiments. PD-L1 expression was significantly suppressed in CCNE1-silenced hepG2, MCF-7, and SK-MEL-28 cells (Fig. 2B and D). The hepG2, MCF-7, and SK-MEL-28 cells were cocultured with macrophages, and the migration of macrophages was significantly inhibited in CCNE1-silenced hepG2, MCF-7, and SK-MEL-28 cells (Fig. 2E and F). The multiplex immunofluorescence staining assay revealed that CCNE1-positive cells were surrounded by a higher number of CD68/CD163-positive cells and CD8-positive cells in tumor tissues than normal tissues in LIHC, BRCA, and SKCM (Fig. 2G).
Fig. 2.
In vitro validation on CCNE1. A. Western blot assay showing CCNE1 expression in hepG2, MCF-7, and SK-MEL-28 cells. B. Western blot assay showing PD-L1 expression in hepG2, MCF-7, and SK-MEL-28 cells. C. Statistical analysis for CCNE1 expression in hepG2, MCF-7, and SK-MEL-28 cells. D. Statistical analysis for PD-L1 expression in hepG2, MCF-7, and SK-MEL-28 cells. E. Coculture Transwell assay showing the migrated macrophages after coculture with hepG2, MCF-7, and SK-MEL-28 cells. F. Statistical analysis for coculture Transwell assay. G. Multiplex immunofluorescence staining of CCNE1, CD68, CD163, and CD8 in liver cancer, breast cancer, and melanoma
Discussion
This study identified the TRPM family members as critical markers across various cancer types. Specifically, TRPM family members appear to play key roles in cancer development and progression through genetic alterations. The developed TRPM-Score demonstrated the ability to predict malignancy and immunological characteristics in pan-cancer effectively. Through a multi-step machine learning and deep learning approach, CCNE1 emerged as a pivotal biomarker related to the TRPM family, with strong predictive value in pan-cancer. CCNE1, a highly conserved cyclin family member, is essential for regulating the cell cycle, particularly in facilitating the G1/S transition. Overexpression of CCNE1 leads to uncontrolled malignant phenotype [9] and therapy resistance [10] of cancer. The functional annotation revealed that CCNE1 predominantly regulates lymphocyte-mediated immunity-related genes (e.g., EXO1, TAP2, IL1B), cell cycle-related genes (e.g., CDK4), p53 signaling pathway-related genes (e.g., CDK1), cellular senescence-related genes (e.g., FOXM1, CDKN2A), as well as genes involved in inflammatory mediator regulation of TRP channels (e.g., IL1B). Immunotherapy has transformed cancer treatment, with continued advancements shaping the field [11]. However, the role of CCNE1 in cancer immunotherapy remains largely unexplored. In our study, CCNE1 expression was correlated with elevated levels of immune checkpoint molecules PD-1, PD-L1, and CTLA-4, along with higher levels of key immunotherapy biomarkers such as TMB, MSI, CYT, and GEP. The tumor microenvironment plays a crucial role in determining the efficacy of anti-cancer immune responses and can contribute to poor outcomes with immunotherapy [12]. Our in vitro experiments confirmed the immunomodulatory functions of CCNE1, showing that its silencing led to reduced PD-L1 expression and impaired macrophage migration. This suggests that CCNE1 may be mediating immune evasion in the tumor microenvironment. Previous in vivo validation proved that CCNE1 is critical for causing chromosome instability in LIHC [13]. Besides, CCNE1 is essential for the development and advancement of liver fibrosis and LIHC [14]. Notably, CCNE1 was found to drive LIHC progression in a CDK2- and kinase-independent manner [15]. Collectively, our findings highlight the TRPM family, particularly TRPM-related CCNE1, as critical players in pan-cancer that may serve as predictive biomarkers and therapeutic targets, especially in the context of immuno-oncology. The comprehensive characterization of TRPM family members and identifying CCNE1 as a key downstream effector represent significant advancements in our understanding of pan-cancer biology.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors express gratitude to the public databases, websites, and software (StrataQuest software (TissueGnostics, Vienna, Austria) and TissueFAXS platform (TissueGnostics, Vienna, Austria)) used in this study.
Abbreviations
- CYT
Cytolytic Activity
- DEG
Differentially Expressed Gene
- GEP
T Cell-Inflamed Signature
- GBM
Glioblastoma
- IFNγ
Interferon-Gamma
- ICB
Immune Checkpoint Blockade
- KIRC
Kidney Clear Cell Carcinoma
- LassoCox
Least Absolute Shrinkage and Selection Operator Regularized Cox Regression
- LassoLR
Least Absolute Shrinkage and Selection Operator Regularized Logistic Regression
- LIHC
Liver Hepatocellular Carcinoma
- MSI
Microsatellite Instability
- NSCLC
Non-Small Cell Lung Cancer
- Pamr
Prediction Analysis for Microarrays
- SCNA
Somatic Copy Number Alteration
- SVM
Support Vector Machine
- SKCM
Skin Cutaneous Melanoma
- STAD
Stomach Adenocarcinoma
- TCR
T Cell Receptor
- TMB
Tumor Mutation Burden
- TNF
Tumor Necrosis Factor
- TRP
Transient Receptor Potential
- TRPC
Transient Receptor Potential Canonical
- TRPV
Transient Receptor Potential Vanilloid
- TRPM
Transient Receptor Potential Melastatin
Author contributions
QC, CH, and ZX conceived, designed, and supervised the study. NZ and HZ performed the data analysis. HZ and SL designed and conducted the experiments. NZ, HZ, and WW wrote the manuscript. QC, CH, ZX, PL, ZL, and YC revised the manuscript. All authors read, edited, and approved the final manuscript.
Funding
This work was supported by the China Postdoctoral Science Foundation (2023MD734131), Chongqing Postdoctoral Science Foundation (CSTB2023NSCQBHX0002), Chongqing Postdoctoral Research Special Funding Project (2023CQBSHTB3095).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
We purchased the pan-cancer tissue array from the Wuhan Tanda Scientific Co., LTD company with ethics approval.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nan Zhang, Hao Zhang, Shuyu Li and Wantao Wu contributed equally to this work.
Contributor Information
Zhiwei Xia, Email: xiazhiwei2011@gmail.com.
Chenshen Huang, Email: chenshenhuang@126.com.
Quan Cheng, Email: chengquan@csu.edu.cn.
References
- 1.Koivisto AP, Belvisi MG, Gaudet R, Szallasi A. Advances in TRP channel drug discovery: from target validation to clinical studies. Nat Rev Drug Discov. 2022;21:41–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhong T, Zhang W, Guo H, Pan X, Chen X, He Q, Yang B, Ding L. The regulatory and modulatory roles of TRP family channels in malignant tumors and relevant therapeutic strategies. Acta Pharm Sin B. 2022;12:1761–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marini M, Titiz M, Souza Monteiro de Araujo D, Geppetti P, Nassini R, De Logu F. TRP channels in Cancer: signaling mechanisms and translational approaches. Biomolecules 2023, 13. [DOI] [PMC free article] [PubMed]
- 4.Hantute-Ghesquier A, Haustrate A, Prevarskaya N. Lehen’kyi V: TRPM Family Channels in Cancer. Pharmaceuticals (Basel) 2018, 11. [DOI] [PMC free article] [PubMed]
- 5.Wu W, Wang X, Liao L, Chen J, Wang Y, Yao M, Zhu L, Li J, Wang X, Chen AF, et al. The TRPM7 channel reprograms cellular glycolysis to drive tumorigenesis and angiogenesis. Cell Death Dis. 2023;14:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosh S, Yang R, Duraki D, Zhu J, Kim JE, Jabeen M, Mao C, Dai X, Livezey MR, Boudreau MW, et al. Plasma membrane Channel TRPM4 mediates Immunogenic Therapy-Induced necrosis. Cancer Res. 2023;83:3115–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chubanov V, Kottgen M, Touyz RM, Gudermann T. TRPM channels in health and disease. Nat Rev Nephrol. 2024;20:175–87. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Vallega KA, Boda VK, Quan Z, Wang D, Fan S, Wang Q, Ramalingam SS, Li W, Sun SY. Targeting transient receptor potential Melastatin-2 (TRPM2) enhances therapeutic efficacy of Third Generation EGFR inhibitors against EGFR Mutant Lung Cancer. Adv Sci (Weinh). 2024;11:e2310126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H, George E, Kinose Y, Kim H, Shah JB, Peake JD, Ferman B, Medvedev S, Murtha T, Barger CJ, et al. CCNE1 copy number is a biomarker for response to combination WEE1-ATR inhibition in ovarian and endometrial cancer models. Cell Rep Med. 2021;2:100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerrero-Zotano A, Belli S, Zielinski C, Gil-Gil M, Fernandez-Serra A, Ruiz-Borrego M, Ciruelos Gil EM, Pascual J, Munoz-Mateu M, Bermejo B, et al. CCNE1 and PLK1 mediate resistance to Palbociclib in HR+/HER2- metastatic breast Cancer. Clin Cancer Res. 2023;29:1557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S, Zhang N, Zhang H, Yang Z, Cheng Q, Wei K, Zhou M, Huang C. Deciphering the role of LGALS2: insights into tertiary lymphoid structure-associated dendritic cell activation and immunotherapeutic potential in breast cancer patients. Mol Cancer. 2024;23:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang T, Huang X, Zhang G, Hong Z, Bai X, Liang T. Advantages of targeting the tumor immune microenvironment over blocking immune checkpoint in cancer immunotherapy. Signal Transduct Target Ther. 2021;6:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aziz K, Limzerwala JF, Sturmlechner I, Hurley E, Zhang C, Jeganathan KB, Nelson G, Bronk S, Fierro Velasco RO, van Deursen EJ, et al. Ccne1 overexpression causes chromosome instability in liver cells and liver Tumor Development in mice. Gastroenterology. 2019;157:210–e226212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otto J, Verwaayen A, Penners C, Hundertmark J, Lin C, Kallen C, Paffen D, Otto T, Berger H, Tacke F, et al. Expression of cyclin E1 in hepatic stellate cells is critical for the induction and progression of liver fibrosis and hepatocellular carcinoma in mice. Cell Death Dis. 2023;14:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geng Y, Michowski W, Chick JM, Wang YE, Jecrois ME, Sweeney KE, Liu L, Han RC, Ke N, Zagozdzon A, et al. Kinase-independent function of E-type cyclins in liver cancer. Proc Natl Acad Sci U S A. 2018;115:1015–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.