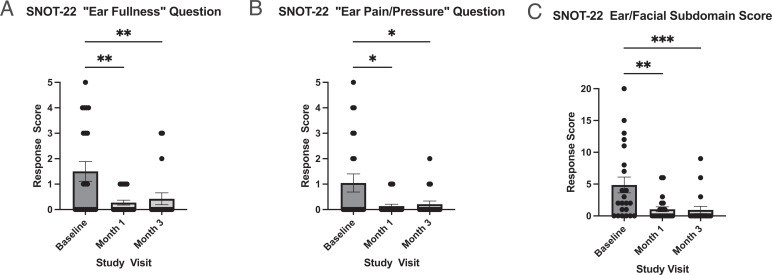
Figure 3.
Decreases in otologic SNOT-22 scores after 1 and 3 months of treatment with dupilumab. The SNOT-22 otologic questions score (range, 0–5) and SNOT-22 ear/facial subdomain score (range, 0–20) 1 month and 3 months after initiating dupilumab are shown. Dupilumab treatment significantly reduced the mean SNOT-22 ear/facial subdomain score (p < 0.001), “ear fullness” (p = 0.001), and “ear pain/pressure” (p = 0.01) response scores (mixed-effects model, n = 22). Columns reflect mean score ± SE, with individual data points plotted. Pairwise comparisons on graph reflect post hoc Tukey multiple comparisons test (*p < 0.05, **p < 0.01, ***p < 0.001). SNOT-22 = 22-Item Sino-Nasal Outcome Test; SE = standard error.