Abstract
Background:
Visceral pain occurs commonly following thoracic surgery, but an effective method to relieve visceral pain in thoracic surgery remains controversial. The authors test the effect of stellate ganglion blocks (SGB) on perioperative visceral pain following video-assisted thoracoscopic surgery (VATS).
Methods:
A prospective, randomized, controlled trial enrolled 77 elderly patients undergoing VATS. Patients were randomized to SGB followed by modified intercostal nerve block (Group S, n=37); or modified intercostal nerve block only (Group C, n=40). Remifentanil 0.02–0.2 μg·kg-1·min-1 was titrated to keep pain threshold index values between 40 and 65 and maintain mean arterial pressure or heart rate values around 20% of baseline values. Patient-controlled intravenous analgesia with sufentanil was used in the postoperative period. The co-primary outcomes were the perioperative cumulative opioid consumption and pain scores on movement at 24 h after surgery.
Results:
Compared with the control group, SGB greatly reduced the intraoperative remifentanil consumption [300.00 (235.00–450.00)μg versus 710.00 (500.00–915.00)μg; P<0.01], with no difference in cumulative sufentanil consumption to 48 h postsurgery. There was a statistically significant difference in pain scores on movement at 24 h between groups [4.00 (3.00–4.00) versus 4.00 (3.25–5.00); P=0.01]. Further exploratory analyses showed a significant difference in intrachest pain on movement at 24 h [3.00 (2.00–3.00) versus 3.00 (2.25–4.00); P=0.01]. No significant difference was observed in nausea/vomiting, time to pass flatus, and postoperative length of stay.
Conclusion:
Preoperative SGB for elderly patients could effectively blunt intraoperative visceral stress and reduce postoperative visceral pain extending 24 h after VATS. This initial finding deserves further investigation.
Keywords: opioid, pain threshold index, stellate ganglion blocks, visceral pain
Introduction
Highlights
Stellate ganglion block could effectively blunt intraoperative visceral stress.
Postoperative visceral pain scores extending 24 h were notably reduced.
However, an accelerated rapid recovery was not observed.
This potentially effective technique is worth exploring for clinical practice.
Visceral pain occurs commonly after thoracic surgery1. It is considered to be the result of the direct stimulation of the pleura or lungs by a drainage tube and referred pain from irritation transmitted via the phrenic nerve. Visceral pain can impair respiration, mobility, and physical therapy in the early postoperative period2. To date, an effective method to relieve visceral pain in thoracic surgery remains controversial, including traditional epidural block alone3,4, paravertebral block5,6, or phrenic nerve block7. Thus, a safe and effective solution for the management of visceral pain is needed.
As a component of multimodal analgesia, the addition of a regional analgesic technique is strongly recommended7 for video-assisted thoracoscopic surgery (VATS). A paravertebral block or erector spinae plane block is recommended as a first-choice option while anterior serratus plane block as a second-choice option. Although traditional intercostal nerves block is not recommended, modified intercostal nerve block (MINB)8 may be a potential alternative method because of the combined effect of the anterior serratus plane and intercostal blocks. Our previous study8 found that MINB was effective in inhibiting nociceptive stress arising from peripheral tissue after VATS. And combination of low-dose dexmedetomidine infusion9 might be an effective alternative method to blunt intraoperative visceral stress, but not involved visceral pain postoperatively. Although phrenic nerve is involved in referred pain, intraoperative phrenic nerve block10 could not be effective for reducing ipsilateral shoulder pain (ISP) from visceral irritation after VATS.
As the stellate ganglion11 provides efferent sympathetic outflow to mediastinum and pleura, stellate ganglion blocks (SGB) was believed to blunt sympathetic and cardiovascular response12. To the best of our knowledge, no study has investigated the usefulness of SGB in VATS. We hypothesized that infiltration of a local anesthetic around the stellate ganglion at the C6 level would be effective for suppressing visceral pain. The aim of this study was to test the effect of SGB on perioperative visceral pain following VATS, through assessment of perioperative opioid consumption and postoperative visceral pain.
Methods
Study design and participants
This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines. This open, randomized, controlled trial was approved by the ethics committee, and written informed consent was obtained from all patients. The trial was registered prior to patient enrollment at clinicaltrials.gov. Patients with American Society of Anesthesiologists(ASA) II–III, aged 60–75 years old, were scheduled to video-assisted thoracoscopic lung cancer surgery from 21 March 2022 to 30 September 2022. The exclusion criteria included those who have contraindications to regional block (allergic to local anesthetics, infection around the puncture site, and coagulation disorders), history of stroke (within 3 months), inability to consent, and history of analgesic dependence. Those who were converted to thoracotomy during the operation, the blood loss was greater than 500 ml and refused to participate in the study were also excluded.
Randomization and blinding
Using a computer-generated random number table, patients were randomized (1:1) to SGB group (group S): stellate ganglion block followed by MINB and control group (group C): MINB alone. MINB9 was defined as a novel approach to achieve the double effect of the anterior serratus plane and intercostal block, using a single injection of a smaller dose of local anesthetic at the fifth intercostal level. While performing SGB, patients in the control group received a blinded sham procedure: had an ultrasound scan and sham puncture with no liquid given. A study coordinator, who was not engaged in patient recruitment, data collection, perioperative care, or postoperative follow-up, sealed the randomization results in sequentially numbered opaque envelopes and kept the envelopes. In the process of the study, the coordinator carried out drug preparation in accordance with the randomization results. The treatment allocation was open to the anesthesiologist performing the stellate ganglion block and induction. However, the anesthesiologist performing the anesthesia management, the study investigators, clinicians, and the patient were blinded to the study group.
SGB
At the preanesthesia room, we placed a high frequency (5–10 MHz) ultrasound probeultrasound probe (S-Nerve, SonoSite Inc.) on the ipsilateral anterior cervical region, a 22G needle was advanced to the C6 vertebra using in-plane short-axis technique and positioned immediately under the prevertebral fascia on the surface of the longus colli muscle. Once the placement of the needle had been confirmed, 5 ml of 0.35% ropivacaine combined with 0.025% dexamethasone was injected on the ipsilateral side of surgery, and a successful stellate ganglion blockade demonstrates specific clinical signs of Horner’s syndrome (ptosis, miosis, anhidrosis, and facial flushing).
MINB
After induction, at the fifth intercostal spaces, close to the lateral side of the surgical incision, a MINB was performed as previously described9. Once the needle tip was advanced to the lower lateral border of the rib, 5 ml of 0.35% ropivacaine combined with 0.025% dexamethasone was administered.
Anesthesia management
All patients were monitored for invasive BP, HR, ECG, and SpO2 after entering the operation room, with a peripheral vein of the upper limb being established. The pain threshold index (PTI) was obtained from the HXD-I multifunction combination monitor (Beijing Easymonitor Technology Co., Ltd.). Baseline blood pressure were recorded after midazolam administration of 0.02 mg·kg-1. Induction of anesthesia was followed by intravenous administration of sufentanil 0.4–0.5 μg·kg-1 and etomidate 0.3 mg·kg-1. And rocuronium 0.6 mg·kg-1 was given to facilitate double-lumen endotracheal intubation. Then one-lung mechanical ventilation was used by VT 6 ml·kg-1, RR 14–16 times·min-1, PEEP 5 cmH2O. Propofol 3–8 mg·kg-1·h-1 was continuously infused to maintain bispectral index 40–60. Remifentanil 0.02–0.2 μg·kg-1·min-1 was titrated to keep PTI values between 40 and 65 and maintain mean arterial pressure (MAP) or heart rate values around 20% of baseline values. Blood gas analysis was monitored intraoperatively to maintain the internal environment and CO2 pressure. Additional single blous of remifentanil 50–100 μg may be administered to inhibit the excitability of the sympathetic nerves during hilar lymphatic dissection and lung expansion flushing. 20 min before the end of the surgery, 0.1 μg·kg-1 sufentanil was used as an analgesic load while connecting to the intravenous patient-controlled analgesia (PCA) pump: sufentanil 0.04 μg·kg-1·h-1 diluted with 0.9% saline to 150 ml, with a background dose of 2 ml·h-1, bolus dose of 2 ml, and an interval of 10 min. After surgery, patients were admitted to the postanesthesia care unit (PACU).
The MAP and HR were continuously measured and recorded before induction (baseline, T0), incision (T1), immediately after entering into the chest (T2), 1 min after exploration (T3), 3 min after exploration (T4), 30 min after surgery (T5), 60 min after surgery (T6), immediately after lung expansion (T7), at the end of surgery (T8). After surgery, the static and dynamic visual analog scale (VAS) scores was assessed at 3 h, 6 h, 12 h, 24 h, 48 h, and 72 h. Bolus dose was given to patients immediately on patient demand. Tramadol was given if the VAS remained more than 3 after two consecutive PCA boluses. The analgesic doses were recorded, and tramadol doses were converted to intravenous sufentanil microgram equivalents (SME) of sufentanil/tramadol potency ratio of 1:500013.
Outcomes
The co-primary outcomes were the perioperative opioid consumption (including intraoperative cumulative remifentanil and postoperative sufentanil consumption) and VAS scores on movement at 24 h after surgery. Secondary outcomes included intraoperative hemodynamics, VAS scores at rest or on movement for incision pain, and intrachest pain during 72 h postoperatively, respectively. Time to first PCIA demand, rescue analgesics to 48 h, nausea/vomiting to 48 h, time to flatus, time to ambulation, and length of hospital stay were also recorded.
Statistical analysis
We planned a sample size of 74 participants (37 per group) to provide 90% power to detect a 50% reduction of cumulative remifentanil consumption in patients receiving stellate ganglion block intervention with a=0.05, based on SD of 100 μg and 220 μg after stellate ganglion block and no intervention, respectively (preliminary observations showed similar postoperative sufentanil consumption between groups). The sample would have been sufficient to exclude pain scores after stellate ganglion block >1 greater than after control intervention at an a threshold 0.0514,15. Allowing for a 10% drop-out rate, we enrolled 41 participants per group.
All data were analyzed using SPSS 22.0 statistical software. Normally distributed measurement data are expressed as mean±SD; single-factor analysis of variance is used, and t-test is used for between-group comparison. Non-normally distributed measurement data are expressed as median and quartile [IQR], using two independent samples of Mann–Whitney U-tests; the χ 2 test was used in enumeration data. Generalized estimating equation (GEE) models were performed to evaluate the trends difference in repeated measures of intrachest pain scores at different time within groups and Bonferroni correction was used to adjust the threshold for tests at multiple time-points (P<0.008). A value of P<0.05 was considered statistically significant.
Results
The CONSORT Flowchart showed that 112 patients were screened for this study. Thirty patients were not eligible, declined to participate, or met an exclusion criterion. Thus, 82 patients were assigned randomly to Group S (n=41) or Group C (n=41). Five patients discontinued intervention because of conversion to open surgery or hemorrhage need transfusion. Finally, 77 patients were analyzed.
Baseline data and surgical characteristics were comparable between groups (Table 1). Notably, the time to extubation and length of PACU in Group S were lower than that in Group C. The co-primary outcomes showed a statistically significant difference (Table 2). Cumulative remifentanil consumption was markedly decreased in Group S compared with the Group C [300.00 (235.00–450.00)μg versus 710.00 (500.00–915.00)μg; median difference=360.00 μg; 95% CI (260.00–460.00)μg; P<0.01). There was a statistically significant difference in pain scores on movement at 24 h between groups [4.00 (3.00–4.00) versus 4.00 (3.25–5.00); median difference=1.00; 95% CI (0–1.00); P=0.01]. Further exploratory analyses showed significant difference for intrachest pain on movement at 24–h [3.00 (2.00–3.00) versus 3.00 2.25–4.00); P=0.01], not for incision pain on movement at 24 h [4.00 (3.00–4.00) versus 4.00 (3.25–5.00); P=0.07]. Although there was no statistical difference in total sufentanil consumption to 48 h after surgery, the time to first PCIA demand in Group S was significantly prolonged compared with Group C [20.00 (18.00–38.50) h versus 11.50 (9.00–21.50) h ; P<0.01], with fewer total number of PCA demands to 48 h [4.00 (2.00–6.00) versus 5.00 (4.00–6.00); P=0.02]. No significant difference was observed in nausea/vomiting, time to pass flatus, and postoperative length of stay.
Table 1.
Demographic data and surgical characteristics.
| Variable | Group C (n=40) | Group S (n=37) | P |
|---|---|---|---|
| Age, years | 68.5 (64.0–72.8) | 67.0 (62.0–70.0) | 0.19 |
| Female sex, n (%) | 12 (30.0%) | 16 (43.2%) | 0.23 |
| BMI (kg/m²) | 22.8 (21.4–23.9) | 22.3 (20.4–24.9) | 0.68 |
| ASA physical status, n (%) | 0.76 | ||
| II | 23 (57.5%) | 20 (54.1%) | |
| III | 17 (42.5%) | 17 (45.9%) | |
| Surgery type, n (%) | 0.11 | ||
| Lobectomy | 35 (87.5%) | 27 (73.0%) | |
| Segmentectomy | 5 (12.5%) | 10 (27.0%) | |
| Duration of anesthesia (min) | 145.0 (114.5–186.3) | 169.0 (110.5–196.0) | 0.98 |
| Duration of surgery (min) | 126.0 (98.5–166.8) | 134.0 (84.5–173.0) | 0.83 |
| Propofol consumption (mg) | 535.0 (452.5–817.5) | 540.0 (400.0–790.0) | 0.80 |
| Time to extubation (min) | 10.0 (8.0–11.8) | 7.0 (5.0–8.0) | <0.01 |
| Length of PACU (min) | 30.0 (30.0–33.8) | 25.0 (19.0–30.0) | <0.01 |
Data are shown as median median (IQR), or number of patients (percentage).
ASA, American Society of Anesthesiologists; Group C, control group; Group S, SGB group; PACU, postanesthesia care unit.
Table 2.
Outcomes after SGB and exploratory analyses for differences.
| Variable | Group C (n=40) | Group S (n=37) | *Hazard ratio or #median difference (95%CI) | P |
|---|---|---|---|---|
| Co-primary outcomes | ||||
| Perioperative opioid consumption | ||||
| Cumulative remifentanil consumption; μg | 710.00 (500.00–915.00) | 300.00 (235.00–450.00) | 360.00 (260.00–460.00)# | <0.01 |
| Cumulative sufentanil consumption to 48 h;μg | 126.84 (112.93–139.78) | 122.30 (107.77–134.2) | 3.99 (−5.03 to 12.90)# | 0.37 |
| Pain scores on movement at 24 h | 4.00 (3.25–5.00) | 4.00 (3.00–4.00) | 1.00 (0.00–1.00)# | 0.01 |
| Secondary outcomes | ||||
| Time to first PCA demand; h | 11.50 (9.00–21.50) | 20.00 (18.00–38.50) | −10.00 (−14.00 to −7.00)# | <0.01 |
| PCA demands to 24 h | 3.00 (1.00–4.00) | 2.00 (0–3.50) | 1.00 (0–2.00)# | 0.04 |
| PCA demands to 48 h | 5.00 (4.00–6.00) | 4.00 (2.00–6.00) | 1.00 (0–2.00)# | 0.02 |
| Rescue analgesics to 48 h | 13 (32.50%) | 8 (21.62%) | 0.57 (0.21–1.60)* | 0.28 |
| Nausea/vomiting to 48 h | 3 (7.50%) | 2 (5.41%) | 0.71 (0.11–4.47)* | 1.00 |
| Time to flatus; h | 18.50 (9.00–22.00) | 17.00 (10.00–21.00) | 1.00 (−2.00 to 5.00)# | 0.49 |
| Time to ambulation; h | 19.50 (17.00–23.00) | 22.00 (19.00–25.00) | −2.00 (−4.00 to 0)# | 0.07 |
| Hospital stay; days | 5.00 (4.00–6.00) | 5.00 (4.00–7.00) | 0 (−1.00 to 1.00)# | 0.96 |
| Exploratory analyses for differences | ||||
| Incision pain on movement at 24 h | 4.00 (3.25–5.00) | 4.00 (3.00–4.00) | 0 (0.00–1.00)# | 0.07 |
| Intrachest pain on movement at 24 h | 3.00 (2.25–4.00) | 3.00 (2.00–3.00) | 1.00 (0.00–1.00)# | 0.01 |
| Moderate or severe pain to 24 h for intrachest pain |
10 (25.00%) | 2 (5.41%) | 0.17 (0.04–0.85)* | 0.02 |
Values are shown as median median (IQR) or number of patients (percentage).
Group C, control group; Group S, SGB group; PCA, patient-controlled analgesia; SGB, stellate ganglion blocks.
No significant difference was observed in incision pain scores at rest or movement within 72 h postoperatively, however, there was a significant reduction in intrachest pain scores at 12 hours (P<0.01) and 24 hours (P<0.05) postoperatively on rest or on at movement (Table 3). GEE models were used to show there was a statistically significant overall trend difference between groups (P=0.02 and P<0.01, respectively, Figure 1).
Table 3.
Postoperative pain scores in patients receiving SGB or control intervention at rest or on movement within 72 h after surgery.
| Group C (n=40) | Group S (n=37) | P | |
|---|---|---|---|
| Incision pain at rest | |||
| 3 h | 0 (0–0) | 0 (0–0) | 0.35 |
| 6 h | 0 (0–1.00) | 0 (0–0.50) | 0.44 |
| 12 h | 2.00 (1.00–2.00) | 2.00 (1.00–2.00) | 0.96 |
| 24 h | 3.00 (2.00–3.00) | 3.00 (2.00–3.00) | 0.16 |
| 48 h | 2.00 (2.00–3.00) | 2.00 (2.00–2.00) | 0.08 |
| 72 h | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 0.91 |
| Incision pain on movement | |||
| 3 h | 0 (0–0) | 0 (0–0) | 0.11 |
| 6 h | 1.00 (1.00–2.00) | 2.00 (1.00–2.00) | 0.16 |
| 12 h | 3.00 (2.00–3.00) | 3.00 (2.00–3.00) | 0.33 |
| 24 h | 4.00 (3.25–5.00) | 4.00 (3.00–4.00) | 0.07 |
| 48 h | 3.00 (3.00–4.75) | 3.00 (3.00–3.00) | 0.12 |
| 72 h | 2.00 (1.25–2.00) | 2.00 (2.00–2.00) | 0.35 |
| Intrachest pain at rest | |||
| 3 h | 0 (0–0) | 0 (0–0) | 0.17 |
| 6 h | 0 (0–0) | 0 (0–0) | 0.28 |
| 12 h | 1.00 (0–1.00) | 0 (0–1.00) | 0.01 |
| 24 h | 1.00 (1.00–2.00) | 1.00 (0.50–1.00) | 0.02 |
| 48 h | 1.00 (1.00–2.00) | 1.00 (1.00–1.00) | 0.06 |
| 72 h | 0 (0–1.00) | 0 (0–1.00) | 0.79 |
| Intrachest pain on movement | |||
| 3 h | 0 (0–0) | 0 (0–0) | 0.09 |
| 6 h | 0 (0–1.00) | 0 (0–0) | 0.02 |
| 12 h | 2.00 (1.00–2.00) | 1.00 (1.00–2.00) | <0.01 |
| 24 h | 3.00 (2.25–4.00) | 3.00 (2.00–3.00) | 0.01 |
| 48 h | 2.50 (2.00–4.00) | 2.00 (2.00–2.00) | 0.05 |
| 72 h | 1.00 (0–1.00) | 1.00 (1.00–1.00) | 0.43 |
Values are median (IQR).
Group C, control group; Group S, SGB group.
Figure 1.
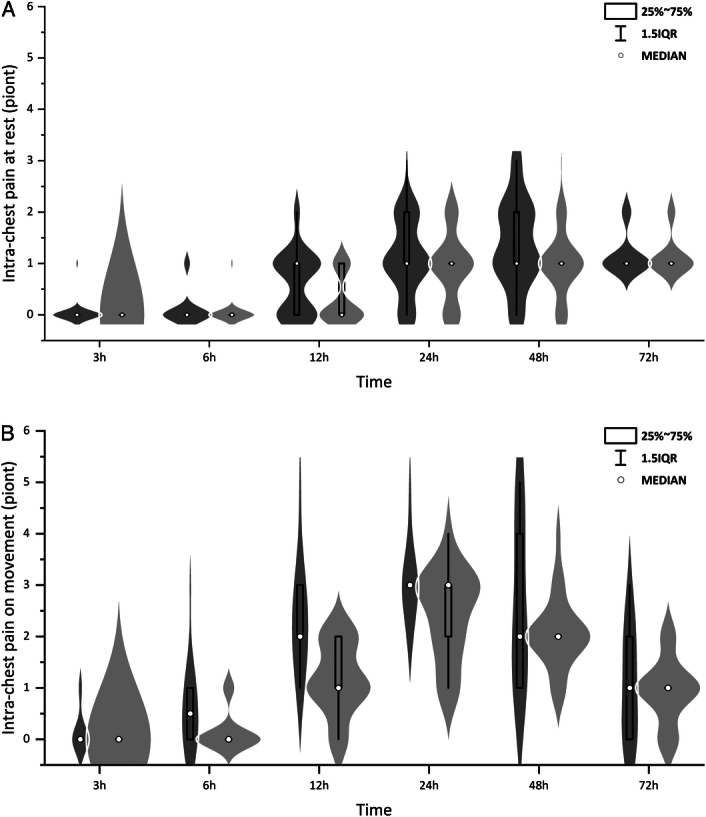
In-chest pain scores at rest (A) or on movement (B) within 72 h were significantly lower in group S (light gray) than that in group C (dark gray), P=0.02 and P=0.00, respectively. Generalized estimating equation(GEE) models were used to correct for multiple testing. The box and dot plots were median (IQR) and outliers (defined as beyond 1.5 times IQR) with the violin plot indicating the density distribution of data. Group S: SGB group; Group C: control group.
MAP and HR in group C immediately after entering into the chest, 1 min, 3 min after exploration and immediately after lung expansion were significantly higher than that in Group S (P<0.01). A similar tendency in MAP and HR before exploration was observed as shown in Figure 2.
Figure 2.
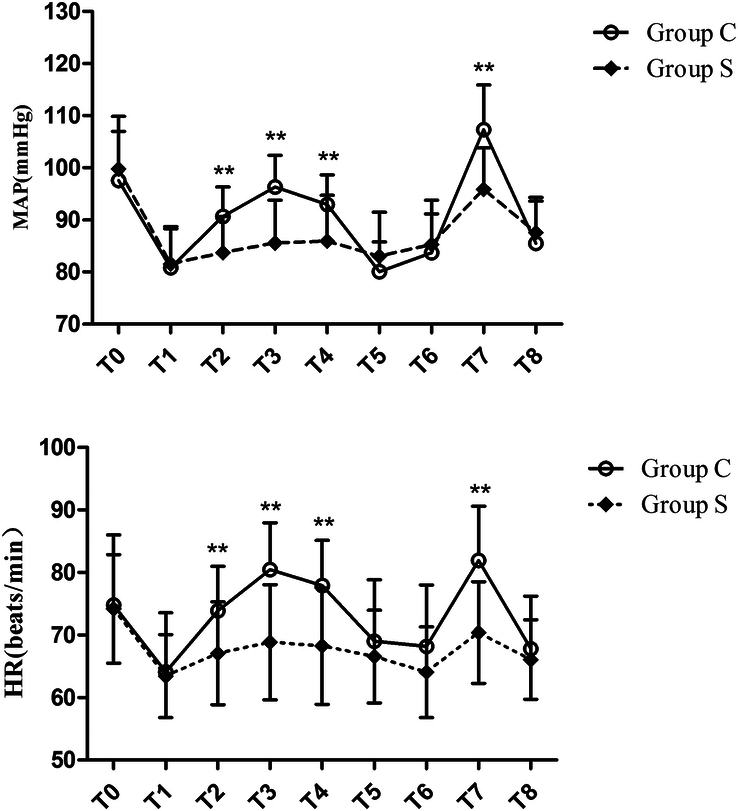
Mean arterial pressure and heart rate changes at different times during surgery. Group C: control group; Group S: SGB group; HR, heart rate; MAP, mean arterial pressure; T0: before induction (baseline); T1: incision; T2: immediately after entering into the chest; T3: 1 min after exploration; T4: 3 min after exploration; T5: 30 min after incision; T6: 60 min after incision; T7: immediately after lung expansion; T8: end of surgery.
Discussion
This study first demonstrated the effect of SGB on the perioperative visceral pain following VATS. Our results found that SGB could effectively blunt intraoperative visceral stress and reduce postoperative visceral pain extending 24 h after surgery. However, SGB could not spare more postoperative opioid consumption and shorten the length of stay.
SGB could inhibit efferent sympathetic nerve activity in the cranial and cervicothoracic areas16. Stellate ganglia play important roles in the regulation of sympathetic nervous activity, and each side of SGB suppresses sympathetic and cardiovascular function17,18. We observed a mild fluctuation in MAP and HR values after SGB intervention once visceral exploration began, especially for lung expansion. Consistent with the previous findings of the antisympathetic effect19,20, we therefore believe that SGB was effective for blunting cardiovascular response originating from excitation of the cervicothoracic sympathetic nerve.
On the other hand, SGB would have blocked not only the efferent sympathetic nerve but also the afferent sensory fibers21. Specific visceral receptors from the pleura, mediastinum and lung root connected to the unmyelinated afferent nerve fibers passing through the stellate ganglion22. Therefore, visceral nociceptive pain23 is believed to be mediated by unmyelinated C-fibers that traverse the sympathetic trunks before entering the spinal cord. More importantly, interneurons of the dorsal horn of the spinal cord are known to have been implicated in referred pain pathways24. However, a standard thoracic epidural analgesia does not provide a total afferent blockade25, some sympathetic fibers might escape the effect of an epidural block26, thus the assertion that visceral afferents from the chest could activate the sympathetic nervous system, which would then exert its effect at the mediastinum, is consistent with the visceral-somatic convergence hypothesis of referred pain24. Since one report27 suggested that stellate ganglion block might be a possible effective treatment, it appears that the sympathetic nervous system may have a role in mediating referred pain. Our findings of an adequate nociceptive control with lower remifentanil consumption suggested SGB might be another suitable alternative to blunt visceral stress well, while MINB8 was effective to relieve the incision pain originating from the incision to the parietal pleura. Furthermore, we also found remifentanil consumption titrated to keep PTI values between 40 and 65 was more than that titrated to keep MAP or heart rate values around 20% of baseline values9.
To our knowledge, blockade of the stellate ganglion is widely used for the management of chronic neuropathic pain28,29. Recent evidence14,15 have verified the role of SGB in the treatment of acute postoperative pain. Since visceral afferents from the chest could activate the sympathetic nervous system, visceral pain after VATS can be seen with sympathetic pain27. Difference from a limited blockade of total afferent fibers after thoracic epidural administration25, we found a significant reduction in intrachest pain VAS at rest or on movement in the SGB group compared with the control group. A plausible explanation is that stellate ganglion is the potential strategy aimed at sympathetic blockade in acute nociceptive pain, which is activated following surgery or injury30, as evidenced by the finding31 of SGB reducing acute postoperative pain in patients following breast cancer. Our study suggested each patient had a fewer total number of PCA demands at the first 48 h. The blocks were effective for up to 24 h postoperatively, which is consistent with a preliminary observation32 of a prolonged duration of analgesia of up to 48 h after SGB. Notably, the median difference of 1 PCA demand in 48 h did not reach the minimal clinically important difference of 10 mg i.v. morphine equivalents for absolute reductions33, although the significant finding of intraoperative remifentanil consumption reached 40% relative reductions33.
The advantage of our study is that this was a rare randomized, controlled study to assess the effect of SGB on visceral pain following thoracoscopic surgery, although the role of SGB was well established in patients with chronic pain. Furthermore, we used PTI monitor to titrate remifentanil consumption, since PTI34 was more accurate than traditional measurement of blood pressure or heart rate. However, there are some limitations. Firstly, patients in control group were not performed with SGB, because of study nature. Although patients in control group received a blinded sham procedure, this may decrease the validity of self-reported outcomes. Notably, this factor did not reduce the power of the trial. Secondly, a background infusion may potentially decrease the effect size in the study group. However, even this, the potential opioid-sparing effect postoperatively did not alter the conclusion. Thirdly, we evaluated intrachest pain other than ISP as a measure of visceral pain, since individual differences for ISP35. Moreover, visceral pain is defined as deep, dull, and more difficult to localize, ISP could not represent whole thoracic visceral pain. Lastly, the sample size is small. Further large-scale trials will be required to strengthen our findings and the potential benefits of SGB in patients following thoracoscopic surgery remains to be determined.
Conclusion
In conclusion, preoperative stellate ganglion block for elderly patients could effectively blunt intraoperative visceral stress and reduce postoperative visceral pain extending 24 h after VATS. However, an accelerated rapid recovery was not observed. These initial findings and the potential benefits of SGB in patients following thoracoscopic surgery deserve further investigation.
Ethical approval
The study protocol was approved by the ethics committee of Zhejiang Cancer Hospital (approval No. IRB-2022-152, Hangzhou, Zhejiang Province, China) and was prospectively registered on the Chinese Clinical Trial Registry (registration number ChiCTR2200057848).
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Source of funding
This work was supported by the Natural Science Foundation of Universities of Anhui Province (No. KJ 2021A0278) and Overseas Study and Research Program for Outstanding Young and Middle-aged Talents in Colleges (gxgwfx2020026).
Author contribution
X.-q.C. and X.-b.X.: designed and conducted the study; X.T. and Y.-l.W.: collected the data; J.Z.: analyzed the data; X.-q.C. and Y.-y.W.: was a major contributor in writing the manuscript; Z.F.: was responsible for revision and correction. All authors approved the final manuscript.
Conflicts of interest disclosure
The authors declare no conflicts of interest.
Research registration unique identifying number (UIN)
ChiCTR2200057848.
Guarantor
Xin-qi Cheng.
Data availability statement
The datasets generated during and analyzed during the current study are available upon reasonable request.
Provenance and peer review
The paper was not invited.
Presentation (for original articles only)
Not applicable.
Acknowledgement
The authors would like to thank all the members of the Thoracic Surgery Department for their support.
Footnotes
Xiao-bing Xiang, Yang-yang Wu, and Zheng Fang contributed equally to this work.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 24 June 2024
Contributor Information
Xiao-bing Xiang, Email: 237607488@qq.com.
Zheng Fang, Email: ay_founder@163.com.
Xiao Tang, Email: 6302270982@qq.com.
Jun Zhou, Email: 181833714@qq.com.
Xin-qi Cheng, Email: ay_mz_cheng@126.com.
References
- 1. Burgess FW, Anderson DM, Colonna D, et al. Ipsilateral shoulder pain following thoracic surgery. Anesthesiology 1993;78:365–368. [DOI] [PubMed] [Google Scholar]
- 2. Li WW, Lee TW, Yim AP. Shoulder function after thoracic surgery. Thorac Surg Clin 2004;14:331–343. [DOI] [PubMed] [Google Scholar]
- 3. Barak M, Ziser A, Katz Y. Thoracic epidural local anesthetics are ineffective in alleviating post-thoracotomy ipsilateral shoulder pain. J Cardiothorac Vasc Anesth 2004;18:458–460. [DOI] [PubMed] [Google Scholar]
- 4. Misiołek H, Karpe J, Copik M, et al. Ipsilateral shoulder pain after thoracic surgery procedures under general and regional anesthesia - a retrospective observational study. Kardiochir Torakochirurgia Pol 2014;11:44–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Helms O, Mariano J, Hentz JG, et al. Intra-operative paravertebral block for postoperative analgesia in thoracotomy patients: a randomized, double-blind, placebo-controlled study. Eur J Cardiothorac Surg 2011;40:902–906. [DOI] [PubMed] [Google Scholar]
- 6. Feray S, Lubach J, Joshi GP, et al. PROSPECT guidelines for video-assisted thoracoscopic surgery: a systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia 2022;77:311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hung YA, Sun CK, Chiang MH, et al. Effect of intraoperative phrenic nerve infiltration on postoperative ipsilateral shoulder pain after thoracic surgeries: a systematic review and meta-analysis of randomized controlled studies. J Cardiothorac Vasc Anesth 2022;36(8 Pt B):3334–3343. [DOI] [PubMed] [Google Scholar]
- 8. Su JJ, Xiang XB, Xu GH, et al. A novel opioid-sparing analgesia following thoracoscopic surgery: a non-inferiority trial. Drug Des Dev Ther 2023;17:1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheng XQ, Cheng J, Zhou YN, et al. Anti-nociceptive effects of dexmedetomidine infusion plus modified intercostal nerve block during single-port thoracoscopic lobectomy: a double-blind, randomized controlled trial. Pain Physic 2021;24:565–572. [PubMed] [Google Scholar]
- 10. Kimura Kuroiwa K, Shiko Y, Kawasaki Y, et al. Phrenic nerve block at the azygos vein level versus sham block for ipsilateral shoulder pain after video-assisted thoracoscopic surgery: a randomized controlled trial. Anesth Analg 2021;132:1594–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guntamukkala M, Hardy PA. Spread of injectate after stellate ganglion block in man: an anatomical study. Br J Anaesth 1991;66:643–644. [DOI] [PubMed] [Google Scholar]
- 12. Chen YQ, Xie YY, Wang B, et al. Effect of stellate ganglion block hemodynamics and stress responses during CO2-pneumoperitoneum in elderly patients. J Clin Anesth 2017;37:149–153. [DOI] [PubMed] [Google Scholar]
- 13. Vercauteren MP, Mertens E, Schols G, et al. Patient-controlled extradural analgesia after caesarean section: a comparison between tramadol, sufentanil and a mixture of both. Eur J Pain 1999;33:205–210. [DOI] [PubMed] [Google Scholar]
- 14. Kumar N, Thapa D, Gombar S, et al. Analgesic efficacy of pre-operative stellate ganglion block on postoperative pain relief: a randomised controlled trial. Anaesthesia 2014;69:954–960. [DOI] [PubMed] [Google Scholar]
- 15. Wen S, Chen L, Wang TH, et al. The efficacy of ultrasound-guided stellate ganglion block in alleviating postoperative pain and ventricular arrhythmias and its application prospects. Neurol Sci 2021;42:3121–3133. [DOI] [PubMed] [Google Scholar]
- 16. Hempel V. The stellate ganglion blockade. Anaesthesist 1993;42:119–128. [PubMed] [Google Scholar]
- 17. Chen YQ, Jin XJ, Liu ZF, et al. Effects of stellate ganglion block on cardiovascular reaction and heart rate variability in elderly patients during anesthesia induction and endotracheal intubation. J Clin Anesth 2015;27:140–145. [DOI] [PubMed] [Google Scholar]
- 18. Song JG, Hwang GS, Lee EH, et al. Effects of bilateral stellate ganglion block on autonomic cardiovascular regulation. Circ J 2009;73:1909–1913. [DOI] [PubMed] [Google Scholar]
- 19. Arai YC, Ogata J, Matsumoto Y, et al. Preoperative stellate ganglion blockade prevents tourniquet-induced hypertension during general anesthesia. Acta Anaesthesiol Scand 2004;48:613–618. [DOI] [PubMed] [Google Scholar]
- 20. Garneau SY, Deschamps A, Couture P, et al. Preliminary experience in the use of preoperative echo-guided left stellate ganglion block in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth 2011;25:78–84. [DOI] [PubMed] [Google Scholar]
- 21. Ikeda T, Iwase S, Sugiyama Y, et al. Stellate ganglion block is associated with increased tibial nerve muscle sympathetic activity in humans. Anesthesiology 1996;84:843–850. [DOI] [PubMed] [Google Scholar]
- 22. Holmes R, Torrance RW. Afferent fibres of the stellate ganglion. Q J Exp Physiol Cogn Med Sci 1959;44:271–281. [DOI] [PubMed] [Google Scholar]
- 23. Cervero F. Visceral nociception: peripheral and central aspects of visceral nociceptive systems. Philos Trans R Soc Lond B Biol Sci 1985;308:325–337. [DOI] [PubMed] [Google Scholar]
- 24. Gebhart GF, Bielefeldt K. Physiology of visceral pain. Compr Physiol 2016;6:1609–1633. [DOI] [PubMed] [Google Scholar]
- 25. Dahl JB, Rosenberg J, Lund C, et al. Effect of thoracic epidural bupivacaine 0.75% on somatosensory evoked potentials after dermatomal stimulation. Reg Anesth 1990;15:73–75. [PubMed] [Google Scholar]
- 26. Kehlet H. Response to surgical stress following thoracic epidural anesthesia. Anesthesiology 1993;78:790–791. [DOI] [PubMed] [Google Scholar]
- 27. Garner L, Coats RR. Ipsilateral stellate ganglion block effective for treating shoulder pain after thoracotomy. Anesth Analg 1994;78:1195–1196. [DOI] [PubMed] [Google Scholar]
- 28. Arnér S, Lindblom U, Meyerson BA, et al. Prolonged relief of neuralgia after regional anesthetic blocks. A call for further experimental and systematic clinical studies. Pain 1990;43:287–297. [DOI] [PubMed] [Google Scholar]
- 29. Price DD, Long S, Wilsey B, et al. Analysis of peak magnitude and duration of analgesia produced by local anesthetics injected into sympathetic ganglia of complex regional pain syndrome patients. Clin J Pain 1998;14:216–226. [DOI] [PubMed] [Google Scholar]
- 30. Bantel C, Trapp S. The role of the autonomic nervous system in acute surgical pain processing – what do we know? Anaesthesia 2011;66:541–544. [DOI] [PubMed] [Google Scholar]
- 31. Yang RZ, Li YZ, Liang M, et al. Stellate ganglion block improves postoperative sleep quality and analgesia in patients with breast cancer: a randomized controlled trial. Pain Ther 2023;12:491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McDonnell JG, Finnerty O, Laffey JG. Stellate ganglion blockade for analgesia following upper limb surgery. Anaesthesia 2011;66:611–614. [DOI] [PubMed] [Google Scholar]
- 33. Laigaard J, Pedersen C, Rønsbo TN, et al. Minimal clinically important differences in randomised clinical trials on pain management after total hip and knee arthroplasty: a systematic review. Br J Anaesth 2021;126:1029–1037. [DOI] [PubMed] [Google Scholar]
- 34. Wu L, Wang S, Wang Y, et al. Prediction of hemodynamic reactivity by electroencephalographically derived pain threshold index in children undergoing general anesthesia: a prospective observational study. J Pain Res 2019;12:3245–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yousefshahi F, Predescu O, Colizza M, et al. Postthoracotomy ipsilateral shoulder pain: a literature review on characteristics and treatment. Pain Res Manag 2016;2016:3652726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and analyzed during the current study are available upon reasonable request.


