Abstract
BACKGROUND:
Worsening renal function (WRF) is common in hospitalized patients being treated for acute heart failure. However, discriminating clinically significant WRF remains challenging. In patients hospitalized with acute heart failure, we evaluated if blood and urine biomarkers of cardiac and kidney dysfunction were associated with adverse outcomes.
METHODS:
We identified 175 of 927 participants in the AKINESIS study (Acute Kidney Neutrophil Gelatinase-Associated Lipocalin Evaluation of Symptomatic Heart Failure Study) who met criteria for stage 1 or 2 Kidney Disease: Improvement Global Outcomes acute kidney injury during the first 3 days of hospitalization. We measured 24 blood and urine biomarkers from specimens collected within 24 hours of meeting acute kidney injury criteria. The primary composite outcome consisted of worsening WRF (higher acute kidney injury stage), need for dialysis, or death at 30 days. Biomarkers’ association with the composite outcome was assessed with logistic regression by tertiles and area under the curve (AUC).
RESULTS:
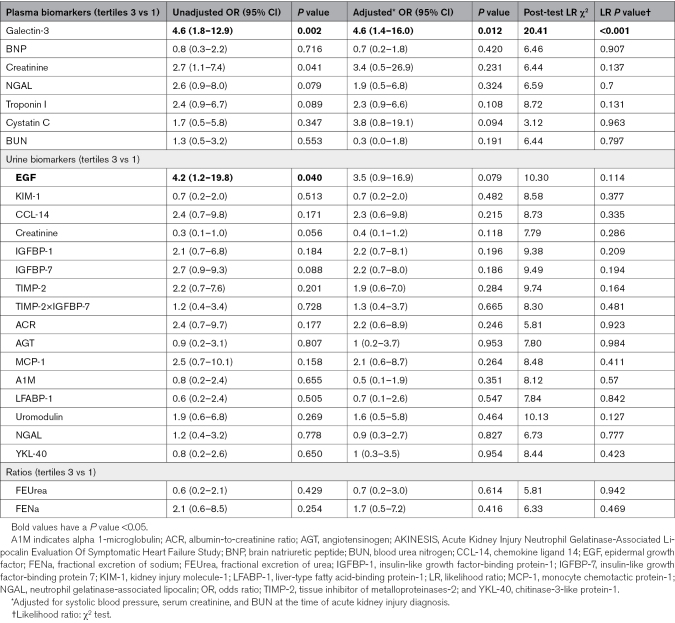
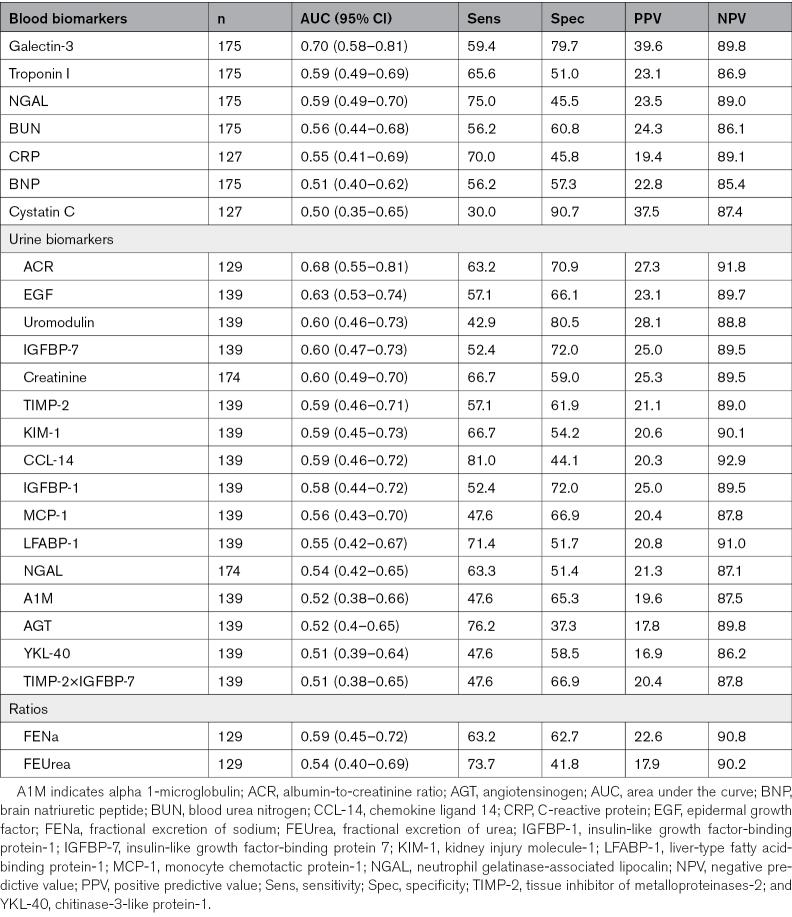
Of the 175 participants, 32 (18%) developed the primary composite outcome. Only history of chronic kidney disease was significantly different between those with and without the composite outcome. The highest tertile of plasma Gal-3 (galectin-3) and urine epidermal growth factor were associated with increased odds of the composite outcome compared with the lowest tertile in unadjusted analyses. After adjusting for serum creatinine, systolic blood pressure, and blood urea nitrogen, only the highest tertile of Gal-3 was associated with greater odds of the composite outcome (odds ratio, 4.6 [95% CI, 1.4–16.0). Gal-3 had the highest AUC (0.70 [95% CI, 0.58–0.82]), while epidermal growth factor had a lower AUC (0.63 [95% CI, 0.53–0.74]). Notably, urine biomarkers of kidney tubule injury were not associated with the composite outcome.
CONCLUSIONS:
Tubular injury does not occur in most patients with acute heart failure experiencing WRF, consistent with the functional mechanisms of WRF in this patient population.
REGISTRATION:
URL: https://www.clinicaltrials.gov/study/NCT01291836?term=NCT01291836&rank=1; Unique identifier: NCT01291836.
Keywords: acute kidney injury, blood pressure, epidermal growth factor, galectin 3, kidney
WHAT IS NEW?
In a prospective observational study, among 24 kidney and cardiac biomarkers, only Gal-3 (galectin-3) and epidermal growth factor were associated with the primary composite outcome of worsening renal failure, death, or renal replacement therapy within 30 days.
Epidermal growth factor was no longer associated with progression to the primary outcome after multivariate adjustment.
WHAT ARE THE CLINICAL IMPLICATIONS?
The lack of association of kidney biomarkers with adverse kidney outcomes suggests that tubular injury does not occur in most patients with worsening renal function in acute heart failure.
Elevations in creatinine are most likely caused by hemodynamic changes, and concurrent adverse outcomes are not a result of tubular injury but likely cardiac or noncardiorenal pathology.
Serum Gal-3 may be able to discriminate high-risk individuals with acute kidney injury and, along with urine epidermal growth factor, warrants further evaluation for the prognostication of worsening renal function in acute heart failure.
See Editorial by Brademeyer and Cox
Worsening renal function (WRF) is common in hospitalized patients being treated for acute heart failure (AHF), but its clinical significance is unclear. Longitudinal increases in serum creatinine are common in AHF, are often the result of reversible hemodynamic changes or drug effects, and are not consistently associated with adverse clinical outcomes when adequate decongestion is achieved concurrently.1–3 However, in the 5% to 15% of patients with AHF without adequate decongestion, the presence of significant tubular injury has been associated with increased mortality.4 This suggests that people with WRF in the setting of AHF represent a heterogeneous group, with many having benign hemodynamic changes in kidney function, while others may have intrinsic kidney injury.
New blood and urine biomarkers that reflect kidney injury or dysfunction within the tubules have been discovered.5 In other settings, these biomarkers strongly predict subsequent loss of kidney function, incident development of heart failure, cardiovascular events, and other adverse clinical events.5–9 However, while some studies have evaluated tubular injury biomarkers for predicting incident WRF and adverse outcomes in AHF, few studies have assessed the role of novel biomarkers for the prediction of WRF progression, and there are limited tools for determining which patients will progress or develop other adverse outcomes.3,10–13 Additionally, there are multiple other novel kidney and cardiac biomarkers reflecting injury, function, inflammation, fibrosis, and repair that have not been evaluated for the progression of WRF in AHF.5
The AKINESIS study (Acute Kidney Injury Neutrophil Gelatinase-Associated Lipocalin Evaluation of Symptomatic Heart Failure Study) is an international multicenter prospective cohort of patients hospitalized with AHF.10 All clinically measured serum creatinine values for the hospitalization were recorded, while blood and urine specimens for biomarker analysis were collected during the first 3 days of hospitalization. In this analysis, we evaluated multiple biomarkers of glomerular and tubular function and injury, in addition to cardiac biomarkers, in the subgroup of patients with AHF with WRF from AKINESIS.
Methods
The study was approved by international review boards at each site, and each patient provided and signed informed consent. On reasonable request, anonymized data may be shared with other researchers.
Study Population
The original study design of AKINESIS has been described previously.10 Briefly, AKINESIS enrolled 927 patients at 16 sites in the United States and Europe. Patients were enrolled if they had findings consistent with AHF and had received or planned receipt of intravenous diuretic therapy. Exclusion criteria were (1) acute coronary syndrome, (2) dialysis dependence or planned initiation during the hospitalization, (3) organ transplantation, (4) enrollment in a drug treatment study within the past 30 days or prior enrollment in AKINESIS, and (5) pregnant or vulnerable populations determined by the institutional review board. Blood and urine specimens were collected and stored at 6 time points. The first specimen was collected on the day of enrollment within 2 hours of the first intravenous diuretic dose. The second specimen was collected 2 to 6 hours later. The third, fourth, and fifth specimens were collected on hospital days 1, 2, and 3, respectively. The sixth specimen was collected on the day of discharge or anticipated discharge. The last 4 collections were not timed with diuretic administration.
In the present analysis, we identified 175 patients meeting the criteria for stage 1 or 2 Kidney Disease: Improving Global Outcomes 2012 acute kidney injury (AKI) creatinine criteria within 72 hours of admission, all of whom had serial serum creatinine measurements available and had stored urine and plasma specimens collected within 24 hours of meeting the WRF criteria.14 Of these, 168 met stage 1 criteria, defined as a serum creatinine increase of ≥0.3 mg/dL within 48 hours or 1.5× increase within 7 days from baseline, and 7 met stage 2 criteria, defined as a serum creatinine increase of 2.0 to 2.9× from baseline.
Laboratory Measurements
For biomarker measurements, between 81.1% and 93.1% of measurements were performed with specimens collected on the day of AKI diagnosis. When a specimen was unavailable on the day of AKI diagnosis, a measurement from a specimen collected on the day before, after, or both times was used, and this value (or average of 2 values) was used. Further details on the missingness of specimens for each biomarker and specimen measurements are provided in the Supplemental Methods (Table S1).
Plasma cystatin C, Gal-3 (galectin-3), high-sensitivity cardiac troponin I, and BNP (B-type natriuretic peptide) were measured with the Architect ci4100 analyzer (Abbott Diagnostic, Wiesbaden, Germany). Plasma neutrophil gelatinase-associated lipocalin was measured with the Alere Triage platform. Biomarkers of plasma CRP (C-reactive protein), sodium (Na), blood urea nitrogen (BUN), and the urinary biomarkers of sodium, urea, and albumin were measured using the Alinity ci-series platform. Fractional extraction of urea and sodium were calculated as previously described.15,16 Biomarkers measured in urine using the Luminex 200 platform (Luminex, Austin, TX) with kits produced by R&D Systems (Minneapolis, MN) included A1M (alpha 1-microglobulin), IGFBP-1 (insulin-like growth factor-binding protein-1), IGFBP-7, KIM-1 (kidney injury molecule-1), MCP-1 (monocyte chemoattractant protein-1), and YKL-40 (chitinase-3-like protein-1).
Urinary biomarkers measured using ELISA included AGT (angiotensinogen), TIMP-2 (tissue inhibitor of metalloproteinases-2), LFABP-1 (liver fatty acid-binding protein-1), UMOD (uromodulin), CCL-14 (C-C motif chemokine ligand 14), and epidermal growth factor (EGF). All ELISA and Luminex assays were conducted in duplicate with blinding to clinical data. Further details of the assays, including the limit of detection and coefficient of variation values, are described in Table S2.
Outcomes
The primary outcome was a composite outcome defined as progression to a higher WRF stage, renal replacement therapy (RRT) requirement, or death within 30 days of hospital admission. We also examined a kidney-specific outcome of WRF progression to a higher WRF stage or RRT within 30 days as a secondary outcome.
Statistical Analysis
Normally distributed continuous variables were expressed as means with SDs; non-normally distributed variables were described as medians and interquartile ranges (IQRs); and categorical variables were described as percentages. Groups were compared using the Student t test, Mann-Whitney U test, or χ2 test comparing progressors versus nonprogressors, as appropriate.
The primary analysis evaluated the association of each biomarker by tertiles on the day of WRF diagnosis with the primary outcome using multivariable logistic regression. In the primary analysis, biomarkers were adjusted for the following clinical variables chosen a priori based on the prior literature: systolic blood pressure, serum creatinine, and serum BUN.17–19 In the secondary analysis, a smaller sample size was available, and biomarkers were adjusted for systolic blood pressure and serum BUN. The added value of a biomarker was determined using the adjusted odds ratio (OR) of the highest versus the lowest biomarker tertile. The discrimination performance of biomarkers as continuous variables was analyzed using the area under the curve (AUC). The optimal biomarker sensitivity and specificity levels were determined using the threshold of the minimum distance from the top-left corner of the AUC curves. In addition, we used the integrated discrimination improvement and the category-free net reclassification index to assess the predictive value that biomarkers add to the clinical models. Urinary biomarkers, except for albumin-to-creatinine ratio (ACR) and fractional excretion biomarkers, were adjusted for urinary creatinine to account for urinary tonicity. All biomarkers were right-skewed and were log2 transformed before analysis. All biomarkers were also assessed for linearity with restricted cubic splines, and only Gal-3 and ACR were found to have nonlinear associations. As a sensitivity analysis, Gal-3 and ACR were modeled as restricted cubic splines using 3 or 4 knots, and the results were compared with those using tertiles.
Analyses were performed using R, version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). Statistical comparisons used a threshold of P<0.05 to determine significance. Additional details are provided in the Supplemental Materials.
Results
Baseline Characteristics
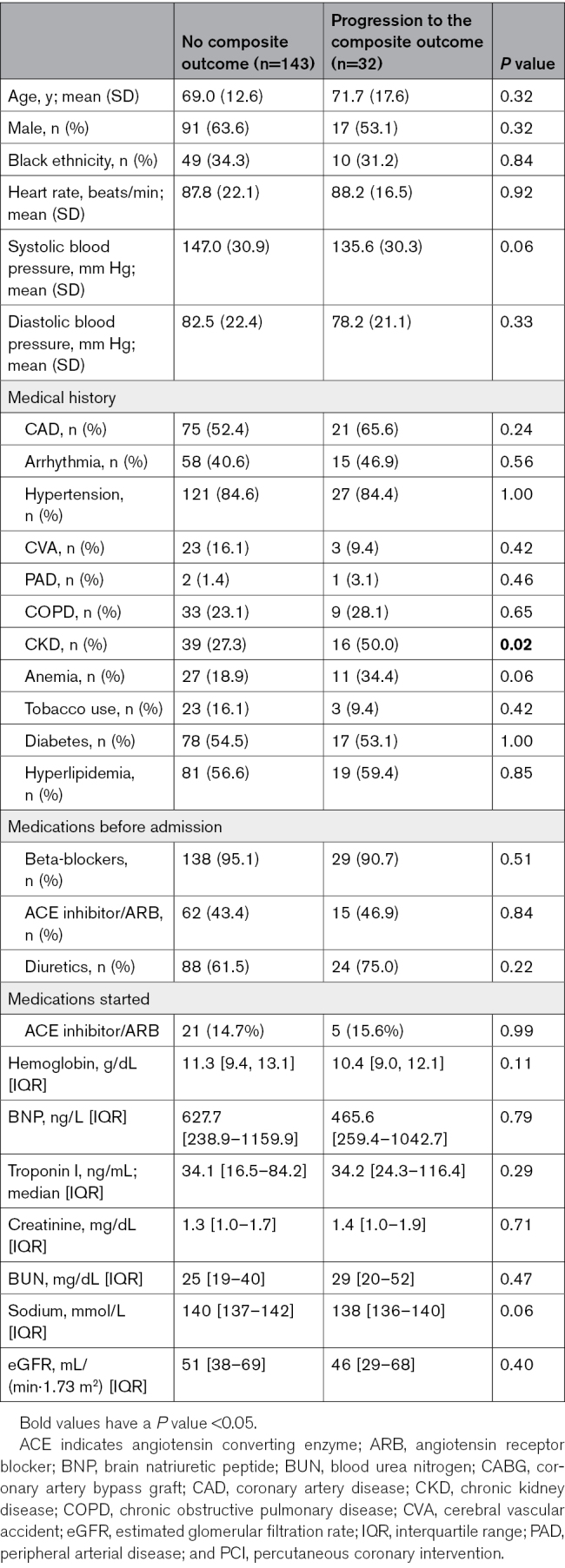
Of the 175 patients with stage 1 or 2 WRF, 32 (18%) developed the primary composite outcome. Twenty-four reached a higher WRF stage, of whom 13 progressed from stage 1 to stage 2, 10 from stage 1 to stage 3, and 1 patient from stage 2 to stage 3. RRT was required in 3 patients, and 14 died within 30 days of admission. Individuals who had the primary composite outcome more often had a reported history of chronic kidney disease (CKD) at baseline (Table 1). They tended to have a history of anemia, lower admission systolic blood pressure, and lower admission serum sodium concentration. Admission estimated glomerular filtration rate and serum BUN were not significantly different between groups. Among the 24 patients who progressed to the secondary outcome of WRF or RRT in 30 days, admission demographics, comorbidities, medications, and baseline laboratory values were similar between groups (Table S3).
Table 1.
Baseline Characteristics in Individuals With and Without the Primary Composite Outcome
Biomarker Levels in Individuals With and Without Outcomes
Of the biomarkers measured at the time of WRF diagnosis, only 2 differed significantly between those with and without the primary composite outcome: plasma Gal-3 with levels of 39.5 ng/mL (IQR, 25.0–63.7 ng/mL) in progressors versus 26.9 ng/mL (IQR, 20.6–34.0 ng/mL) in nonprogressors (P<0.001), and urinary EGF with levels of 14.2 ug/g Cr (IQR, 6.7–31.9 ug/g Cr) in progressors versus 6.6 ug/g Cr (IQR, 2.3–19.6 ug/g Cr; P=0.049) in nonprogressors were significantly different (Table S4; Figure S1). All other blood and urine biomarkers were similar between those with and without the primary composite outcome. Similarly, plasma Gal-3 and urinary EGF were significantly higher in those who progressed to the secondary outcome versus nonprogressors (Table S5; Figure S2).
Prognostic Utility of Biomarkers for the Primary Composite Outcome
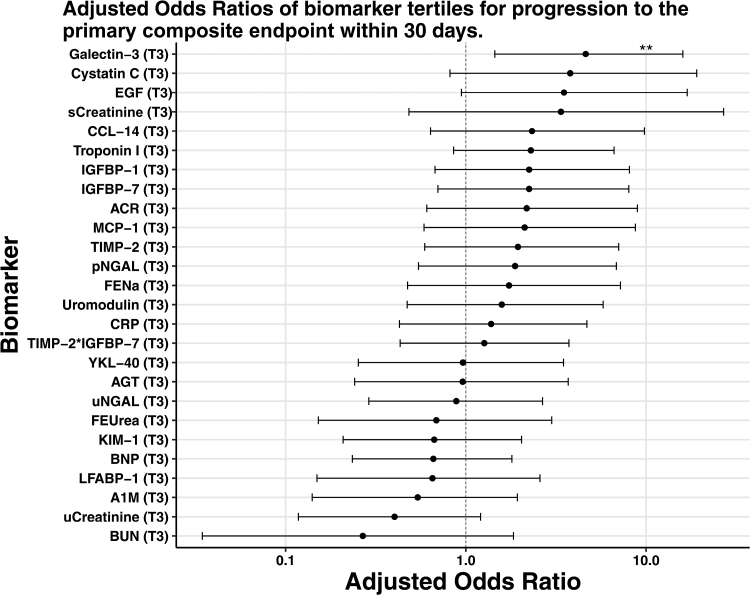
In a multivariable logistic regression model adjusting for SBP, serum creatinine, and BUN at the time of WRF, only plasma Gal-3 was significantly associated with higher odds of the primary composite outcome (OR, 4.6 [95% CI, 1.4–16] for the highest versus the lowest biomarker tertile; Table 2; Figure). The highest tertile of urinary EGF was associated with higher odds of WRF progression in unadjusted analysis (OR, 4.2 [95% CI, 1.2–19.8]; P=0.040); however, while the association was of similar magnitude, it was no longer significant after adjusting for SBP, serum creatinine, and BUN (OR, 3.5 [95% CI, 0.9–16.9]; P=0.079). When plasma Gal-3 and urine ACR were modeled with restricted cubic splines in a sensitivity analysis, the results were similar to those described above.
Table 2.
Odds of Developing the Primary Composite Outcome of Progression to a Higher Stage of Worsening Renal Function, Renal Replacement Therapy, or Death Within 30 d in the Highest vs the Lowest Tertile of Biomarkers in Unadjusted and Adjusted Logistic Regression in Individuals With Stage 1 or 2 Acute Kidney Injury in the AKINESIS Study
Figure.
Adjusted odds ratios of the highest tertile vs lowest biomarker tertile for progression to the primary composite end point within 30 days. Odds ratio are adjusted for systolic blood pressure, serum creatinine and BUN. **P<0.05. A1M indicates alpha 1-microglobulin; ACR, albumin-to-creatinine ratio; AGT, angiotensinogen; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CCL-14, chemokine ligand 14; CRP, C-reactive protein; EGF, epidermal growth factor; FENa, fractional excretion of sodium; FEUrea, fractional excretion of urea; IGFBP-1, insulin-like growth factor-binding protein-1; IGFBP-7, insulin-like growth factor-binding protein 7; KIM-1, kidney injury molecule-1; LFABP-1, liver-type fatty acid-binding protein-1; MCP-1, monocyte chemotactic protein-1; pNGAL, plasma neutrophil gelatinase-associated lipocalin; sCreatinine, serum creatinine; TIMP-2, tissue inhibitor of metalloproteinases-2; uCreatinine, urinary creatinine; uNGAL, urine neutrophil gelatinase-associated lipocalin; and YKL-40, chitinase-3-like protein-1.
When we evaluated the secondary outcome of progression to a higher stage of WRF or RRT, higher plasma Gal-3 was the only biomarker associated with greater odds of this event (OR, 3.5 [95% CI, 1.1–12.4]; P=0.041; Table S6; Figure S3) after adjusting for SBP, serum creatinine, and BUN.
Biomarker Discrimination for Outcomes
Plasma Gal-3 had good discrimination for development of the primary composite outcome with an AUC of 0.70 (95% CI, 0.58–0.82; Table 3). Plasma Gal-3 performed best as a negative predictor with a NPV of 89.8% versus a PPV of 39.6%. Urine EGF had fair discrimination with an AUC of 0.63 (95% CI, 0.53–0.74). This biomarker also performed better for its negative predictive value than its positive predictive value. Urine ACR also had fair discrimination with an AUC of 0.68 (95% CI, 0.55–0.81). The other biomarkers studied had poor to fair AUCs of 0.6 or lower (Table 3).
Table 3.
Plasma and Urine Kidney Tubule Injury and Function Biomarkers Performance for the Discrimination of the Primary Composite Outcome of Progression to Higher Stage of Worsening Renal Function, Renal Replacement Therapy, or Death at the Time of Developing Worsening Renal Function by Area Under the Curve, Sensitivity, and Specificity in Hospitalized Patients With Acute Heart Failure
Plasma Gal-3 was the only biomarker to improve reclassification for both the category-free net reclassification index and integrated discrimination improvement (Table S7). The significant improvement in category-free net reclassification index (0.52 [95% CI, 0.14–0.89]; P=0.007) was driven by strong prediction of nonevents with a net reclassification index for nonevents of 0.33 (95% CI, 0.17–0.48; P<0.001). Plasma troponin I, urinary TIMP-2, and urinary IGFBP-7 also reclassified individuals from having the primary composite outcome to not having it, as seen in the significant net reclassification index for events for these biomarkers. Only urinary LFABP-1 was able to correctly reclassify individuals from not having WRF progression to having WRF progression.
Discussion
The pathogenesis and outcomes for WRF in AHF are heterogeneous. While most individuals experience WRF due to hemodynamic or drug effects (eg, diuretics and vasodilators), some individuals have intrinsic kidney injury and ongoing worsening of kidney function that increases the risk for other adverse events. As a glomerular filtration marker, serum creatinine cannot distinguish these 2 causes of WRF, as it will longitudinally increase in both scenarios. In this analysis of 175 patients with AHF who developed WRF within 72 hours of hospital admission, we evaluated 24 blood and urine biomarkers of cardiac and kidney pathophysiology to determine whether they were associated with risk of progressing to a higher stage of WRF, need for RRT, or death in 30 days above and beyond creatinine-based kidney function at the time of WRF diagnosis. Of the urine biomarkers, we found only the highest tertile of urine EGF was associated with progression of WRF in unadjusted analysis, but this association was attenuated and no longer significant after adjusting for SBP, serum creatinine, and BUN. Of blood biomarkers, the highest tertile of plasma Gal-3 was significantly associated with a higher odds of WRF progression in unadjusted and adjusted analyses. Our findings from a range of novel cardiorenal biomarkers in a large and well-characterized cohort reaffirm that WRF in AHF is largely a functional syndrome, with a lack of kidney tubular injury in most cases.
While early studies suggested WRF in AHF was associated with adverse outcomes, multiple studies have shown the majority of WRF results from hemodynamically mediated functional changes such as blood pressure lowering, congestion with venous hypertension, alterations in glomerular efferent and afferent arteriolar tone, decongestion, hemoconcentration, and use of medications that impact kidney blood flow.1,2,20,21 These processes exert hemodynamic effects on the glomerulus and filtration of serum creatinine, but do not necessarily lead to injury of the kidney tubules. In AHF, as long as adequate decongestion is achieved, it appears that WRF is not strongly associated with adverse outcomes at the group level.1,2,20,21 However, 5% to 15% of patients in these studies experience WRF without an obvious hemodynamic cause for WRF and are at the greatest risk of death and HF readmission. This has prompted research to find potentially clinically undetected kidney processes, such as tubular injury or dysfunction or acute cardiac dysfunction or injury changes that may both relate to WRF progression and to a less effective response to decongestion therapies, driving these outcomes. Prior work by our group and others on a limited number of tubular injury biomarkers did not show any substantial tubular injury in the interval before WRF diagnosis.3,10,22 An important limitation of this prior work was the limited number of biomarkers evaluated, making it possible that other biomarkers may provide different insights. In addition, from a clinical perspective, the treating physician would most likely wish to distinguish hemodynamic versus other causes of WRF when serum creatinine is increasing, that is, when the WRF becomes clinically manifest. Our analysis here confirms these prior findings but goes further by evaluating an extensive list of kidney tubular injury biomarkers that have proven utility in other clinical settings like sepsis, critical care, and kidney transplant and clearly demonstrating no difference in values or any prognostic utility for identifying progression of WRF in AHF.5,6,23–27 We think our findings present the most definitive evidence that WRF in AHF is not from tubular injury in most cases but primarily from functional systemic and glomerular hemodynamic processes. It is also likely that nonkidney pathophysiology drives morbidity and mortality in those patients experiencing WRF without adequate decongestion. Future research should focus on other pathophysiologic processes that lead to worse outcomes in the subset of patients with AHF and WRF who do not achieve adequate decongestion.
While tubular injury was not associated with adverse kidney outcomes, plasma Gal-3 was the only biomarker that consistently discriminated between individuals who developed versus did not develop the primary and secondary outcomes. Plasma Gal-3 is involved in multiple physiological processes and is important for cardiorenal pathophysiology; it has been linked to kidney tubular development and to inflammation and fibrosis in both the heart and kidney.28–30 Studies have shown an association between higher plasma Gal-3 and incident CKD, CKD progression, AKI, AKI severity, and AKI progression in different clinical settings.31–33 However, plasma Gal-3 is also increased in people with CKD, perhaps due to retention from a lower estimated glomerular filtration rate.34 Interestingly, in our analyses, plasma Gal-3 retained its association with ongoing WRF despite adjusting for serum creatinine and BUN at the time of WRF diagnosis. In a translational study using a mouse model of AKI, antagonism of plasma Gal-3 attenuated cardiac fibrosis and abhorrent remodeling, opening up the possibility that excretion of plasma Gal-3 by the kidney in humans plays an important role in the progression of kidney disease and AKI in humans.35 More recently, urine Gal-3 levels have been found to be associated with a higher risk of adverse outcomes in patients with heart failure and CKD.36 While evidence is mounting for the potential use of Gal-3, both blood and urine measurements, in cardiorenal syndrome, we are cautious in interpreting the data in this cohort, which may be a chance finding, given the large number of biomarkers assessed without correction for multiple comparisons.
Beyond plasma Gal-3, only urine EGF suggested potential utility for predicting increased risk of the primary composite outcome. EGF is thought to reflect kidney tubule regeneration and repair specifically within the loop of Henle and distal tubule. Along with urine UMOD, urine EGF is unique among the studied urine biomarkers, as lower rather than higher urine levels have been associated with adverse outcomes in other settings.37,38 This provides reassurance that these biomarkers do not simply reflect nonspecific protein loss into the urine. The ability of urine EGF to discriminate the composite outcome in AHF may be related to its relationship with the site of action of loop diuretics. Thus, while our findings clearly showed a lack of tubular injury with WRF in AHF, tubular dysfunction may be present and influence outcomes. EGF has previously been reported to be highly correlated with estimated glomerular filtration rate, and in situ mRNA levels correlated strongly with urinary biomarker levels.39 In AKI survivors, urine EGF levels were recently found to be associated with a higher incidence of major adverse kidney events.40 These findings are hypothesis-generating but suggest that EGF may warrant further research as a tubular injury/repair biomarker, although this was not found in adjusted analyses of our cohort, which lacked evidence of acute tubular injury.
Limitations
Our study has important limitations. Our sample size and event rate were relatively small, as we focused only on patients experiencing WRF during AHF who then went on to worsening WRF. This also limited the extent to which we could adjust for confounders in models. CKD rates were higher in progressors and is a well-recognized risk factor for WRF. Adjustment variables were chosen a priori, and serum creatinine and BUN strongly correlate with CKD status. We chose to limit the primary outcome to those outcomes of clinical significance and not broaden the criteria with potentially less meaningful outcomes. By testing 24 biomarkers, there is a chance of type-1 error; however, in the context of prior studies supporting the association of Gal-3 with prognosis in cardiorenal syndrome, we think this finding is plausible. Although kidney biopsy is the gold standard for defining AKI pathogenesis, it is not routinely performed in AKI, and we think that the diverse array of noninvasive urinary biomarkers of renal tubular stress and damage suggests that the presence of acute tubular injury in patients with AKI in the setting of AHF is not a typical finding. Finally, our negative findings for fractional excretion of sodium and urea should be cautiously interpreted because blood and urine specimens were not timed to diuretic administration.
Conclusions
Tubular injury does not occur in most patients with WRF in AHF. Elevations in creatinine are caused by hemodynamic changes, and concurrent adverse outcomes are not a result of tubular injury but likely cardiac or noncardiorenal pathology. Plasma Gal-3 may be able to discriminate high-risk individuals with WRF, and urine EGF warrants further evaluation for the prognostication of WRF in AHF.
ARTICLE INFORMATION
Acknowledgment
Contributing author Sagar Raturi, PhD, died in August 2024.
Sources of Funding
Dr Wettersten and this study was supported (or supported in part) by Career Development Award IK2 CX002105 from the US Department of Veterans Affairs Clinical Sciences R&D Service. The contents do not represent the views of the US Department of Veterans Affairs or the US Government.
Dr Duff was supported by a Newman Fellowship in Acute Kidney Injury from the University College Dublin Foundation.
Disclosures
Dr Maisel previously received grant funding from Abbott Laboratories and Alere Inc. He is the cofounder of Aseptiscope and Imperium. Dr Côté receives consulting fees from GlaxoSmithKline. Dr Ix holds an investigator-initiated research grant from the National Institute of Diabetes and Digestive and Kidney Diseases. He has served on the advisory boards of Akebia, AstraZeneca, and Bayer. He has received travel support from Kidney Disease: Improving Global Outcomes.
Dr Murray previously received research funding from Abbott Laboratories and Alere Inc. His institution currently receives research grant funding from Abbott. He receives consulting fees from Novartis, AM-Pharma, and Alexion for serving on clinical trial steering committees. He receives consulting fees from Renibus Therapeutics, Calcimedica, and Bioporto Diagnostics for serving on scientific advisory boards. The other authors report no conflicts.
Supplemental Material
Supplemental Methods
Tables S1–S7
Figures S1–S3
Supplementary Material
Nonstandard Abbreviations and Acronyms
- A1M
- alpha 1-microglobulin
- ACR
- albumin-to-creatinine ratio
- AGT
- angiotensinogen
- AHF
- acute heart failure
- AKINESIS
- Acute Kidney Injury Neutrophil Gelatinase-Associated Lipocalin Evaluation Of Symptomatic Heart Failure Study
- AUC
- area under the curve
- BNP
- B-type natriuretic peptide
- BUN
- blood urea nitrogen
- CCL-14
- C-C motif chemokine ligand 14
- CKD
- chronic kidney disease
- CRP
- C-reactive protein
- EGF
- epidermal growth factor
- Gal-3
- galectin-3
- IGFBP
- insulin-like growth factor-binding protein
- KIM-1
- kidney injury molecule-1
- LFABP-1
- liver-type fatty acid-binding protein-1
- MCP-1
- monocyte chemotactic protein-1
- OR
- odds ratio
- RRT
- renal replacement therapy
- TIMP-2
- tissue inhibitor of metalloproteinases-2
- UMOD
- uromodulin
- WRF
- worsening renal function
- YKL-40
- chitinase-3-like protein-1
For Sources of Funding and Disclosures, see pages 1071 and 1072.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCHEARTFAILURE.123.011751.
Contributor Information
Stephen Duff, Email: stephenduff@gmail.com.
Nicholas Wettersten, Email: nwettersten@health.ucsd.edu.
Yu Horiuchi, Email: yooouyou@gmail.com.
Dirk J. van Veldhuisen, Email: d.j.van.veldhuisen@umcg.nl.
Sagar Raturi, Email: mr.sagarraturi@gmail.com.
Ruairi Irwin, Email: r.irwin1987@gmail.com.
Jean Maxime Côté, Email: jean-maxime.cote@umontreal.ca.
Alan Maisel, Email: asmaisel@gmail.com.
References
- 1.Wettersten N, Horiuchi Y, van Veldhuisen DJ, Mueller C, Filippatos G, Nowak R, Hogan C, Kontos MC, Cannon CM, Mueller GA, et al. B-type natriuretic peptide trend predicts clinical significance of worsening renal function in acute heart failure. Eur J Heart Fail. 2019;21:1553–1560. doi: 10.1002/ejhf.1627 [DOI] [PubMed] [Google Scholar]
- 2.McCallum W, Tighiouart H, Testani JM, Griffin M, Konstam MA, Udelson JE, Sarnak MJ. Acute kidney function declines in the context of decongestion in acute decompensated heart failure. JACC: Heart Failure. 2020;8:537–547. doi: 10.1016/j.jchf.2020.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad T, Jackson K, Rao VS, Tang WHW, Brisco-Bacik MA, Chen HH, Felker GM, Hernandez AF, O’Connor CM, Sabbisetti VS, et al. Worsening renal function in acute heart failure patients undergoing aggressive diuresis is not associated with tubular injury. Circulation. 2018;137:2016. doi: 10.1161/CIRCULATIONAHA.117.030112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horiuchi Y, Wettersten N, van Veldhuisen DJ, Mueller C, Filippatos G, Nowak R, Hogan C, Kontos MC, Cannon CM, Müeller GA, et al. Decongestion, kidney injury and prognosis in patients with acute heart failure. Int J Cardiol. 2022;354:29–37. doi: 10.1016/j.ijcard.2022.02.026 [DOI] [PubMed] [Google Scholar]
- 5.Ix JH, Shlipak MG. The promise of tubule biomarkers in kidney disease: a review. Am J Kidney Dis. 2021;78:719–727. doi: 10.1053/j.ajkd.2021.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duff S, Irwin R, Cote JM, Redahan L, McMahon BA, Marsh B, Nichol A, Holden S, Doran P, Murray PT. Urinary biomarkers predict progression and adverse outcomes of acute kidney injury in critical illness. Nephrol Dial Transplant. 2022;37:1668–1678. doi: 10.1093/ndt/gfab263 [DOI] [PubMed] [Google Scholar]
- 7.Jotwani V, Katz R, Ix JH, Gutiérrez OM, Bennett M, Parikh CR, Cummings SR, Sarnak MJ, Shlipak MG. Urinary biomarkers of kidney tubular damage and risk of cardiovascular disease and mortality in elders. Am J Kidney Dis. 2018;72:205–213. doi: 10.1053/j.ajkd.2017.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bullen AL, Katz R, Jotwani V, Garimella PS, Lee AK, Estrella MM, Shlipak MG, Ix JH. Biomarkers of kidney tubule health, CKD progression, and acute kidney injury in SPRINT (Systolic Blood Pressure Intervention Trial) Participants. Am J Kidney Dis. 2021;78:361–368.e1. doi: 10.1053/j.ajkd.2021.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostermann M, Karsten E, Lumlertgul N. Biomarker-based management of AKI: fact or fantasy? Nephron. 2022;146:295–301. doi: 10.1159/000518365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maisel AS, Wettersten N, van Veldhuisen DJ, Mueller C, Filippatos G, Nowak R, Hogan C, Kontos MC, Cannon CM, Muller GA, et al. Neutrophil gelatinase-associated lipocalin for acute kidney injury during acute heart failure hospitalizations: the AKINESIS study. J Am Coll Cardiol. 2016;68:1420–1431. doi: 10.1016/j.jacc.2016.06.055 [DOI] [PubMed] [Google Scholar]
- 11.Murray PT, Wettersten N, van Veldhuisen DJ, Mueller C, Filippatos G, Nowak R, Hogan C, Kontos MC, Cannon CM, Mueller GA, et al. Utility of urine neutrophil gelatinase-associated lipocalin for worsening renal function during hospitalization for acute heart failure: primary findings of the urine N-gal acute kidney injury N-gal evaluation of symptomatic heart failure study (AKINESIS). J Card Fail. 2019;25:654–665. doi: 10.1016/j.cardfail.2019.05.009 [DOI] [PubMed] [Google Scholar]
- 12.Rao VS, Ahmad T, Brisco-Bacik MA, Bonventre JV, Wilson FP, Siew ED, Felker GM, Anstrom KK, Mahoney DD, Bart BA, et al. Renal effects of intensive volume removal in heart failure patients with preexisting worsening renal function. Circ Heart Fail. 2019;12:e005552. doi: 10.1161/circheartfailure.118.005552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C, Yang X, Lei Y, Zha Y, Liu H, Ma C, Tian J, Chen P, Yang T, Hou FF. Urinary biomarkers at the time of AKI diagnosis as predictors of progression of AKI among Patients with acute cardiorenal syndrome. Clin J Am Soc Nephrol. 2016;11:1536–1544. doi: 10.2215/CJN.00910116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. doi: 10.1038/kisup.2011.32 [Google Scholar]
- 15.Kaplan AA, Kohn OF. Fractional excretion of urea as a guide to renal dysfunction. Am J Nephrol. 1992;12:49–54. doi: 10.1159/000168417 [DOI] [PubMed] [Google Scholar]
- 16.Espinel CH. The FENa test. Use in the differential diagnosis of acute renal failure. JAMA. 1976;236:579–581. doi: 10.1001/jama.236.6.579 [DOI] [PubMed] [Google Scholar]
- 17.Klein L, Massie BM, Leimberger JD, O’Connor CM, Piña IL, Adams KF, Califf RM, Gheorghiade M. Admission or changes in renal function during hospitalization for worsening heart failure predict postdischarge survival. Circ Heart Fail. 2008;1:25–33. doi: 10.1161/circheartfailure.107.746933 [DOI] [PubMed] [Google Scholar]
- 18.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Boscardin WJ; ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–580. doi: 10.1001/jama.293.5.572 [DOI] [PubMed] [Google Scholar]
- 19.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J; ADHERE Scientific Advisory Committee and Investigators. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422–430. doi: 10.1016/j.cardfail.2007.03.011 [DOI] [PubMed] [Google Scholar]
- 20.Damman K, Valente MA, Voors AA, O’Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35:455–469. doi: 10.1093/eurheartj/eht386 [DOI] [PubMed] [Google Scholar]
- 21.Testani JM, Coca SG, McCauley BD, Shannon RP, Kimmel SE. Impact of changes in blood pressure during the treatment of acute decompensated heart failure on renal and clinical outcomes. Eur J Heart Fail. 2011;13:877–884. doi: 10.1093/eurjhf/hfr070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray PT, Wettersten N, van Veldhuisen DJ, Mueller C, Filippatos G, Nowak R, Hogan C, Kontos MC, Cannon CM, Mueller GA, et al. Utility of urine neutrophil gelatinase-associated lipocalin for worsening renal function during hospitalization for acute heart failure: primary findings for urine N-gal acute kidney injury N-gal evaluation of symptomatic heart failure study (AKINESIS). J Card Fail. 2019;25:654. doi: 10.1016/j.cardfail.2019.05.009 [DOI] [PubMed] [Google Scholar]
- 23.Garimella PS, Lee AK, Ambrosius WT, Bhatt U, Cheung AK, Chonchol M, Craven T, Hawfield AT, Jotwani V, Killeen A, et al. Markers of kidney tubule function and risk of cardiovascular disease events and mortality in the SPRINT trial. Eur Heart J. 2019;40:3486–3493. doi: 10.1093/eurheartj/ehz392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ix JH, Katz R, Bansal N, Foster M, Weiner DE, Tracy R, Jotwani V, Hughes-Austin J, McKay D, Gabbai F, et al. Urine fibrosis markers and risk of allograft failure in kidney transplant recipients: a case-cohort ancillary study of the FAVORIT trial. Am J Kidney Dis. 2017;69:410–419. doi: 10.1053/j.ajkd.2016.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jotwani V, Scherzer R, Abraham A, Estrella MM, Bennett M, Cohen MH, Nowicki M, Sharma A, Young M, Tien PC, et al. Association of urine alpha1-microglobulin with kidney function decline and mortality in HIV-infected women. Clin J Am Soc Nephrol. 2015;10:63–73. doi: 10.2215/CJN.03220314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park M, Katz R, Shlipak MG, Weiner D, Tracy R, Jotwani V, Hughes-Austin J, Gabbai F, Hsu CY, Pfeffer M, et al. Urinary markers of fibrosis and risk of cardiovascular events and death in kidney transplant recipients: the FAVORIT trial. Am J Transplant. 2017;17:2640–2649. doi: 10.1111/ajt.14284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schrauben SJ, Shou H, Zhang X, Anderson AH, Bonventre JV, Chen J, Coca S, Furth SL, Greenberg JH, Gutierrez OM, et al. ; CKD Biomarkers Consortium and the Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. Association of multiple plasma biomarker concentrations with progression of prevalent diabetic kidney disease: findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. J Am Soc Nephrol. 2021;32:115–126. doi: 10.1681/ASN.2020040487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winyard PJ, Bao Q, Hughes RC, Woolf AS. Epithelial galectin-3 during human nephrogenesis and childhood cystic diseases. J Am Soc Nephrol. 1997;8:1647–1657. doi: 10.1681/ASN.V8111647 [DOI] [PubMed] [Google Scholar]
- 29.Bichara M, Attmane-Elakeb A, Brown D, Essig M, Karim Z, Muffat-Joly M, Micheli L, Eude-Le Parco I, Cluzeaud F, Peuchmaur M, et al. Exploring the role of galectin 3 in kidney function: a genetic approach. Glycobiology. 2006;16:36–45. doi: 10.1093/glycob/cwj035 [DOI] [PubMed] [Google Scholar]
- 30.Slack RJ, Mills R, Mackinnon AC. The therapeutic potential of galectin-3 inhibition in fibrotic disease. Int J Biochem Cell Biol. 2021;130:105881. doi: 10.1016/j.biocel.2020.105881 [DOI] [PubMed] [Google Scholar]
- 31.Tan KCB, Cheung CL, Lee ACH, Lam JKY, Wong Y, Shiu SWM. Galectin-3 is independently associated with progression of nephropathy in type 2 diabetes mellitus. Diabetologia. 2018;61:1212–1219. doi: 10.1007/s00125-018-4552-z [DOI] [PubMed] [Google Scholar]
- 32.Boutin L, Legrand M, Sadoune M, Mebazaa A, Gayat E, Chadjichristos CE, Dépret F. Elevated plasma Galectin-3 is associated with major adverse kidney events and death after ICU admission. Crit Care. 2022;26:13. doi: 10.1186/s13054-021-03878-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iacoviello M, Di Serio F, Rizzo C, Leone M, Grande D, Guida P, Gioia MI, Parisi G, Leopizzi T, Caldarola P, et al. Association between high Gal-3 serum levels and worsening of renal function in chronic heart failure outpatients. Biomarkers Med. 2019;13:707–713. doi: 10.2217/bmm-2018-0349 [DOI] [PubMed] [Google Scholar]
- 34.Zamora E, Lupón J, de Antonio M, Galán A, Domingo M, Urrutia A, Troya M, Bayes-Genis A. Renal function largely influences Galectin-3 prognostic value in heart failure. Int J Cardiol. 2014;177:171–177. doi: 10.1016/j.ijcard.2014.09.011 [DOI] [PubMed] [Google Scholar]
- 35.Prud’homme M, Coutrot M, Michel T, Boutin L, Genest M, Poirier F, Launay JM, Kane B, Kinugasa S, Prakoura N, et al. Acute kidney injury induces remote cardiac damage and dysfunction through the galectin-3 pathway. JACC Basic Transl Sci. 2019;4:717–732. doi: 10.1016/j.jacbts.2019.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao VS, Ivey-Miranda JB, Cox ZL, Moreno-Villagomez J, Testani JM. Association of urine galectin-3 with cardiorenal outcomes in patients with heart failure. J Card Fail. 2023;30:340–346. doi: 10.1016/j.cardfail.2023.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norvik JV, Harskamp LR, Nair V, Shedden K, Solbu MD, Eriksen BO, Kretzler M, Gansevoort RT, Ju W, Melsom T. Urinary excretion of epidermal growth factor and rapid loss of kidney function. Nephrol Dial Transplant. 2021;36:1882–1892. doi: 10.1093/ndt/gfaa208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menez S, Moledina DG, Thiessen-Philbrook H, Wilson FP, Obeid W, Simonov M, Yamamoto Y, Corona-Villalobos CP, Chang C, Garibaldi BT, et al. ; TRIKIC Consortium Investigators. Prognostic significance of urinary biomarkers in patients hospitalized with COVID-19. Am J Kidney Dis. 2022;79:257–267.e1. doi: 10.1053/j.ajkd.2021.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ju W, Nair V, Smith S, Zhu L, Shedden K, Song PXK, Mariani LH, Eichinger FH, Berthier CC, Randolph A, et al. ; ERCB, C-PROBE, NEPTUNE, and PKU-IgAN Consortium. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med. 2015;7:316ra193. doi: 10.1126/scitranslmed.aac7071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menez S, Wen Y, Xu L, Moledina DG, Thiessen-Philbrook H, Hu D, Obeid W, Bhatraju PK, Ikizler TA, Siew ED, et al. The ASSESS-AKI study found urinary epidermal growth factor is associated with reduced risk of major adverse kidney events. Kidney Int. 2023;104:1194–1205. doi: 10.1016/j.kint.2023.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]