Figure 1.
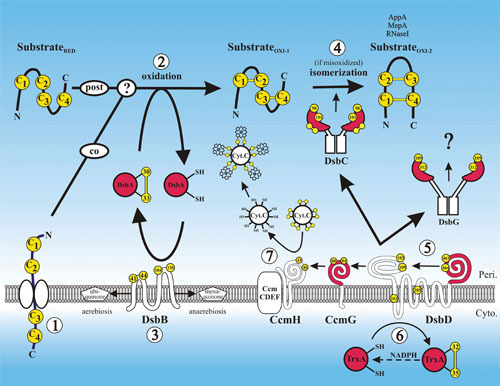
Disulfide bond formation in the periplasm. A protein requiring disulfide bonds for its stability is translocated into the periplasm via the SecYEG translocon with its cysteines (arbitrarily labeled C1 to C4) in a reduced state (substrateRED) (1). Disulfide bond formation is catalyzed by DsbA, either during translocation, after translocation, or both (2). DsbA is reoxidized back to its active oxidized state by DsbB, and DsbB is oxidized by ubiquinone in aerobic conditions or by menaquinone in anaerobic conditions (3). If the substrate is misoxidized (substrateOXI-1), its disulfide bonds are isomerized to their native oxidized states (substrateOXI-2) by DsbC (4). DsbC along with DsbG and CcmG are maintained in their active reduced states by DsbD (5). DsbD in turn is reduced by the cytoplasmic thioredoxin TrxA, which receives its reducing potential ultimately from cytoplasmic pools of NADPH (6). CcmG maintains CcmH in a reduced state. Through the interaction of CcmH with the CcmCDEF membrane complex, oxidized cytochrome-c is reduced, enabling it to form thioether covalent bonds with its heme cofactor (7). Proteins with thioredoxin folds are in red, and cysteines are in yellow. The amino acid residue numbers of the redox-active cysteines are indicated.
