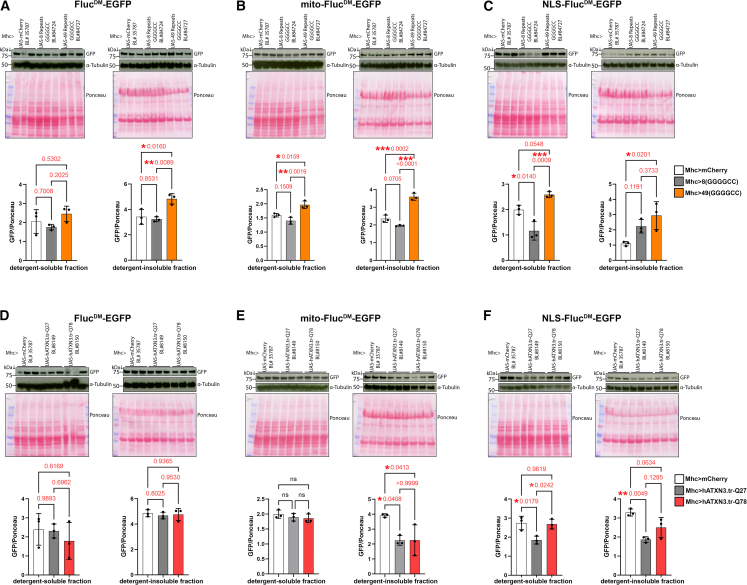
Figure 6.
Distinct aggregation-prone proteins differentially impact subcellular proteostasis
(A–C) Analyses of detergent-soluble and insoluble fractions from skeletal muscle from 10-day-old flies that express a toxic, aggregation-prone protein with 49(GGGGCC) repeats (orange) compared to non-toxic controls, 8(GGGGCC)-containing proteins (gray), and/or mCherry (white). Western blots with anti-GFP antibodies detect the levels of FlucDM-EGFP, whereas Ponceau staining and α-tubulin are used as normalization controls. The toxic 49(GGGGCC) protein increases the detergent-insoluble levels of FlucDM variants that localize to the cytoplasm (A), mitochondria (B), and the nucleus (C), indicating that aggregation-prone proteins with 49(GGGGCC) repeats generally disrupt proteostasis across multiple cell compartments. n = 3 (biological replicates) with the mean ± SD indicated; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (one-way ANOVA).
(D–F) Western blots of detergent-soluble and -insoluble fractions from skeletal muscles of flies that express pathogenic ataxin-3 with poly-glutamine tract expansion (hATXN3.tr-Q78; red) versus a non-pathogenic ataxin-3 (hATXN3.tr-Q27, gray) and mCherry controls (white). There is no modulation of detergent-soluble and -insoluble FlucDM levels (D), whereas the effects of hATXN3.tr-Q78 on mito-FlucDM (E) and NLS-FlucDM (F) levels are inconsistent when compared to the hATXN3.tr-Q27 versus the mCherry control. n = 3 (biological replicates) with the mean ± SD indicated; ∗p < 0.05, ∗∗p < 0.01 (one-way ANOVA). Altogether, these findings indicate that distinct aggregation-prone proteins have strikingly different impacts on subcellular proteostasis.