Abstract
Introduction
We encountered a case of adenocarcinoma of the ectopic pancreas, causing intussusception.
Case presentation
A 76-year-old man presented with complaints of abdominal distention and vomiting to the emergency department in March 2022. Computed tomography showed that the small bowel piled up approximately 20 cm from the ligament of the traits. Endoscopic repair was challenging; therefore, laparoscopic repair and partial resection of the small bowel were performed. The specimen showed a mass in the small bowel arising from an ectopic pancreas that had caused accumulation. Pathological examination revealed ectopic pancreatic cancer. Two years postoperatively, no apparent recurrence has been observed. We report a relatively rare case of a cancerous ectopic jejunal pancreas causing a mass, with a discussion in the literature.
Conclusions
Detection typically requires surgery due to advanced-stage intestinal obstruction or accumulation, as observed in the present case. However, preoperative diagnosis and early detection of ectopic pancreatic cancer are challenging. The disease progresses similarly to pancreatic cancer, highlighting the need for early detection methods. Additionally, accumulating more case reports is essential for establishing an effective treatment strategy.
Keywords: Adenocarcinoma of ectopic pancreas in the jejunum, Small bowel intussusception, Adenocarcinoma
Introduction
Here, we report a case of adenocarcinoma of the ectopic pancreas that caused intussusception.
Reports of carcinomatosis of ectopic pancreatic cancer are rare [1], and the only reported cases discovered as a result of bowel accumulation were our self-examined cases.
Case presentation
A 76-year-old man visited another hospital with chief complaints of abdominal pain and distention. The patient had no specific medical or family history. On examination, a mass was palpable in the upper abdomen. He was referred to our hospital for surgical treatment after a thorough examination using abdominal X-ray and ultrasonography (Fig. 1). Simple computed tomography (CT) revealed an intussusception in the jejunum. Therefore, we performed a contrast-enhanced CT scan (Fig. 2), which showed a preserved contrast effect on the bowel wall. Staging laparoscopy was performed since non-obstructive repair via gastrointestinal endoscopy was difficult.
Fig. 1.
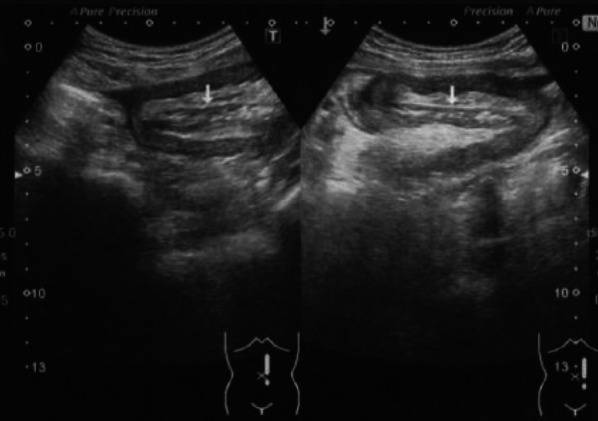
Abdominal ultrasonography. A small amount of ascites in the cysto-rectal fossa, a high echogenic mesentery, and small intestinal insertions in the intestinal tract of the left abdomen can be noted. Oral bowel dilatation was also observed
Fig. 2.
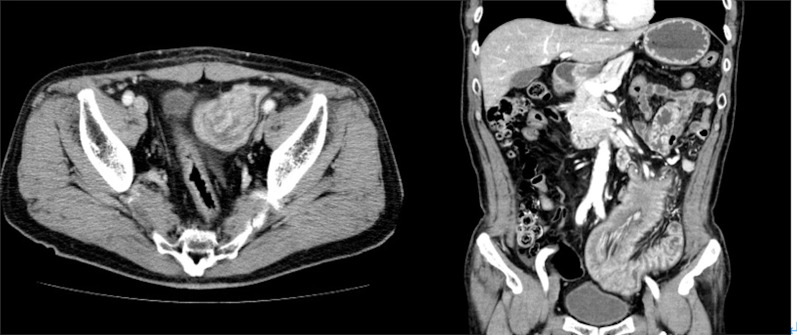
Abdominal contrast-enhanced computed tomography. The image shows a 20-cm-long small bowel–small bowel type superimposed findings with a preceding mass with sparse contrast in the jejunum. No apparent pancreatic abnormalities were observed
A small bowel-type accumulation was observed approximately 10–30 cm from the ligament of Treitz (Fig. 3). The accumulation was easily released by the Hutchinson maneuver using laparoscopic forceps. No ischemic necrosis was found in the area of the small bowel where the accumulation had occurred. The lesion was removed from the body, and an elastic hard mass was identified on palpation of the bowel. Partial small bowel resection was performed with a 10 cm margin before and after the mass. Next, the specimen was opened to confirm the presence of the mass, which was a yellow elevated submucosal tumor 3 cm in size with a relatively firm, uneven, and irregular mucosal lesion suspected to be small bowel cancer. The surgery was completed with additional dissection of the small bowel mesentery in the D2 resected area.
Fig. 3.
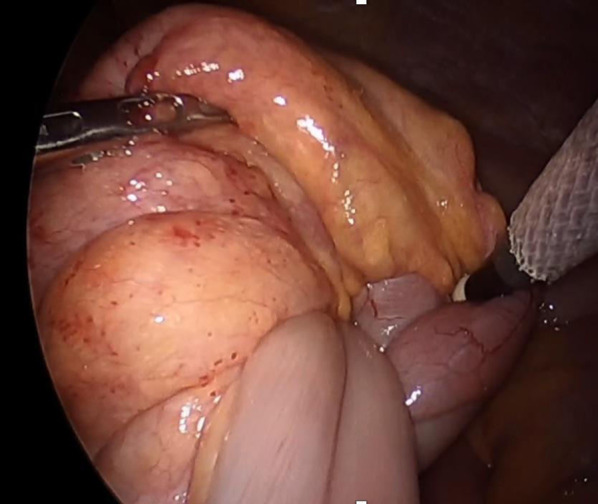
Intraoperative findings. The accumulation was easily released by the Hutchinson maneuver using laparoscopic forceps. No ischemic necrosis was noted in the area of the small bowel where the accumulation had occurred
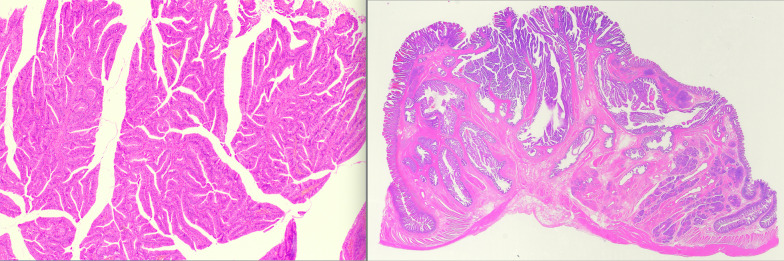
Pathological diagnosis (Figs. 4, 5) revealed pancreatic ducts and acinar cells with no islets of Langerhans, indicating Heinrich classification type II [2]. Tumor cells predominantly proliferated from the submucosa of the jejunum to the intrinsic muscularis mucosae. Simultaneously, an abrupt transition (conflict between normal and neoplastic epithelia) of the normal glandular ducts and malignant findings on the mucosal surface was noted. Originally, no findings in the pancreas were suggestive of the primary tumor.
Fig. 4.
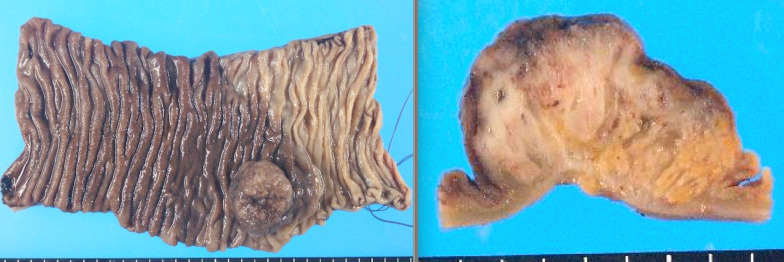
Macroscopic findings. The lesion was diagnosed as invasive ductal carcinoma, papillary adenocarcinoma, arising from the ectopic pancreas of the jejunum. The specifics included INFα, depth mp, ly0, v1, n0, with a tumor diameter of 20 × 20 mm
Fig. 5.
Pathological findings. Pathological diagnosis revealed pancreatic ducts and acinar cells with no islets of Langerhans, indicating Heinrich classification type II. Tumor cells predominantly proliferated from the submucosa of the jejunum to the intrinsic muscularis mucosae. Simultaneously, an abrupt transition (conflict between normal and neoplastic epithelia) of the normal glandular ducts and malignant findings on the mucosal surface was noted
The ectopic pancreatic cancer was classified as pT2N0M0 stage I according to the Union for International Cancer Control-Tumur, Node, Metastasis (UICC-TNM classification, 8th edition, 2017) [3]. According to the National Cancer Center and Rare Cancer Center website [4], resection of the lesion is the primary treatment, and we successfully resected it laparoscopically. Postoperative adjuvant chemotherapy for adenocarcinoma of the small intestine is currently undergoing phase III clinical trials. According to the treatment guidelines for pancreatic cancer, the patient underwent R0 surgery at stage I although postoperative adjuvant chemotherapy was necessary. We recommended S-1 as a first choice; however, he chose not to pursue it. Fortunately, the patient has survived without a recurrence for 2 years.
Discussion
The most common cause of ectopic pancreas is generally believed to be stray pancreatic tissue during fetal development. In addition, the most common sites of ectopic pancreas are the duodenum and stomach, which are close to the pancreas, followed by the jejunum, ileum, and Meckel’s diverticulum [5]. The condition predominantly affects males, with a male-to-female ratio of 2:1. Typically, ectopic pancreas does not present with clinical symptoms. Moreover, Heinrich classification [2] is a well-known tissue classification method. Type I has islets of Langerhans, adenocytes, and conduits and has the same structure as normal pancreatic tissue; type II lacks islets of Langerhans and comprises adenocytes and conduits; type III lacks islets of Langerhans and adenocytes and comprises only conduits; and type IV comprises only islets of Langerhans without pancreatic exocrine tissue. Based on pathological findings, our case was classified as Heinrich type II.
Although multiple reports of ectopic pancreatic carcinogenesis exist in the past, our review of the Non-Profit Organization (NPO) Japan Medical Abstracts Society database from 2000 to 2023 under the terms “jejunum” and “ectopic pancreatic cancer” revealed 22 reports, including our cases. However, the only reported cases discovered as a result of bowel accumulation were our self-examined cases (excluding conference proceedings).
The criteria for the transformation of the ectopic pancreas into carcinoma, according to Mibayashi et al. [6], are as follows: (1) the lesion must be observed grossly, mainly from the submucosa to the muscularis mucosae, and show no association with the mucosa except for mucosal perforation; (2) the ectopic pancreas must be complicated or coexist, and the tumor must show evidence of a transition from these lesions to carcinoma or the histological picture of the tumor must resemble that of pancreatic cancer; and (3) the tumor must not have metastasized from pancreatic cancer. In this case, the patient was found to have intestinal accumulation, and the intraoperative specimen was opened due to suspicion of jejunal carcinoma exposed on the mucosa. Therefore, the lymph nodes of the patient were dissected.
In this case, the pathological findings showed a Heinrich type II ectopic pancreas and a tumorigenic image of the pancreatic duct, mainly in the submucosal layer, extending from the abrupt transition part of the mucosal surface to the intrinsic muscular layer. As the contrast-enhanced CT scan showed a normal pancreas, we considered that the case met the definition of ectopic pancreatic cancer of the jejunum. The size was 3.5 cm, depth was in the intrinsic muscular layer, and no lymph node metastases were observed. According to previous literature, 80% of lesions are 1–3 cm in size, and most of them are located from the submucosa to the muscularis propria [7]. When diagnosed with ectopic pancreatic cancer, Hirata et al. [8] recommended treatment according to pancreatic cancer based on histological features. The need for lymph node dissection, in addition to lesion resection, has been highlighted.
According to the National Cancer Center and Rare Cancer Center website [4], small intestine cancer generally refers to duodenal, jejunal, and ileal cancers, with neuroendocrine tumors, adenocarcinomas, malignant lymphomas, and sarcomas (gastrointestinal stromal tumors and leiomyosarcoma) as the most common histologic types. It is considered a rare type of cancer, accounting for < 0.5% of all malignant tumors and < 5% of all malignant gastrointestinal tumors. Small intestinal adenocarcinoma is staged according to the UICC-TNM classification, although its treatment has not yet been established. While resection of the lesion is a curative treatment, the need for dissection and the resecting area is yet to be clearly elucidated. Moreover, the effectiveness of adjuvant chemotherapy after radical surgery has not been established, and no drugs are covered by insurance. The efficacy of fluoropyrimidine + oxaliplatin therapy has been demonstrated in stage IV patients with distant metastases or recurrent disease, and fluorouracil, leucovorin, and oxaliplatin (FOLFOX) is also covered by insurance in Japan. However, only approximately 10% of a relatively high rate of MSI-positive patients are known, and the efficacy of pembrolizumab has been demonstrated.
Hoshino et al. [1] reported 20 cases of ectopic pancreatic cancer of the jejunum. A total of 22 cases exist, including our case. Among these, eight cases had lymph node metastasis or distant metastasis at surgery, and eight experienced recurrence within 1 year after radical resection, all with a poor prognosis. All patients were treated with chemotherapy according to the guidelines for pancreatic cancer treatment. In addition to the study by Miyazaki et al. [9], eight cases of so-called N0 and M0 have been reported, as in the present case, although the exact staging was difficult to determine as the depth of some cases was unknown. Moreover, the optimal duration of chemotherapy administration is unknown. Table 1 presents the postoperative recurrence and survival of these seven patients with early-stage cancer, with or without adjuvant chemotherapy. Our patient did not receive postoperative adjuvant chemotherapy but survived 24 months postoperatively without recurrence.
Table 1.
Past reported cases of adenocarcinoma of ectopic pancreas in the jejunum
| Case | Authors | Year | Age | Sex | Heinrich type | Metastasis | Adjuvant | Postoperative course |
|---|---|---|---|---|---|---|---|---|
| 1 | Fujiki [12] | 1990 | 54 | M | I | Liver | MTX/5-FU | Death in 7 months |
| 2 | Sato [13] | 1993 | 64 | M | I | Lymph node | Unknown | Recurrence peritoneal in 3 months |
| 3 | Saegusa [14] | 1995 | 76 | F | I | None | Unknown | No recurrence for 14 months |
| 4 | Arao [15] | 1999 | 63 | M | II |
Liver Lymph node |
Unknown | Unknown |
| 5 | Uemura [16] | 2001 | 72 | M | II | Lymph node | Unknown | No recurrence in 6 months |
| 6 | Matsubara [17] | 2004 | 79 | M | Unknown | None | None | Peritoneal recurrence in 6 months and death in 9 months |
| 7 | Sato [18] | 2007 | 78 | F | II | Unknown | Unknown | Unknown |
| 8 | Koh [19] | 2009 | 50 | M | Unknown |
Peritoneum Greater omentum |
None | Peritoneal recurrence in 2 weeks and death in 6 weeks |
| 9 | Ono [20] | 2010 | 51 | F | III | Unknown | UFT/GEM | Peritoneal recurrence in 7 months and death in 28 months |
| 10 | Nagai [21] | 2014 | 70 | M | II | Peritoneum | GEM | Death in 4 months |
| 11 | Okada [22] | 2014 | 71 | M | I | None | GEM/S-1 | Liver recurrence in 10 months |
| 12 | Kurata [23] | 2014 | 70 | M | I | Unknown | Unknown | Unknown |
| 13 | Ogawa [24] | 2015 | 70 | M | I | None | GEM/S-1 | No recurrence in 22 months |
| 14 | Matsuyama [25] | 2015 | 72 | F | II | Peritoneum | None | Death in 1 month |
| 15 | Ito [26] | 2016 | 66 | F | II | None | S-1 | No recurrence in 3 months |
| 16 | Miyazaki [9] | 2016 | 72 | M | I | None | S-1 | No recurrence in 24 months |
| 17 | Yogi [27] | 2017 | 82 | M | I | Unknown | S-1 | No recurrence in 7 months |
| 18 | Sakoda [28] | 2018 | 91 | M | Unknown | None | None | Death in 4 months |
| 19 | Shigemasa [29] | 2019 | 96 | F | II | Unknown | None | Death in 6 months |
| 20 | Sakurai [30] | 2019 | 69 | F | unknown | Peritoneum | GnP | No recurrence in 6 months |
| 21 | Hosino [1] | 2022 | 82 | M | II | None | None | Liver recurrence in 2 months |
| 22 | Our case | 2023 | 76 | M | II | None | None | No recurrence in 24 months |
M, male; F, female; GEM, gemcitabine; GnP, gemcitabine and nab-paclitaxel; MTX/5-FU, methotrexate/5-fluorouracil
S-1 and gemcitabine (GEM) are recommended as adjuvants for pancreatic cancer [10, 11]. As mentioned above, no clear evidence of small intestinal cancer exists. If jejunal ectopic pancreatic cancer is treated as pancreatic cancer, S-1 or GEM would be the treatment of choice. Miyazaki et al. [9] suggested the efficacy of adjuvant S-1 as well as pancreatic cancer. In our case, adjuvant therapy was indicated as the treatment of choice because the pathological findings revealed a tumor with vascular invasion. Although we recommended S-1 as a first choice, we decided to follow up without adjuvant therapy as per the patient’s choice. Fortunately, the disease has not recurred, although we are still carefully monitoring the patient’s progress, partly due to the abovementioned reasons.
In the case of advanced pancreatic cancer, other options are available in accordance with the guidelines for pancreatic cancer treatment; however, the prognosis is extremely poor, even in the cases reported to date. Therefore, as with pancreatic cancer, early detection of ectopic pancreatic cancer is required; however, similar to tumors of the small intestine, it takes time for symptoms to appear. Previously, many patients visited hospitals with abdominal pain and nausea, and intestinal obstruction was found upon close examination. In our case, the patient had a stacked intestine, although the cause of vomiting associated with dilatation of the oral intestinal tract was the same.
Conclusions
For detection, the patient must undergo surgery owing to intestinal obstruction at an advanced stage or intestinal accumulation, as observed in the present case, and because distinguishing ectopic pancreatic cancer in the preoperative diagnosis or early detection is challenging. The disease progresses similarly to pancreatic cancer, making early detection methods crucial. Therefore, accumulating more case reports in the future is necessary to establish an effective treatment strategy.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Abbreviations
- CT
Computed tomography
- UICC-TNM
Union for International Cancer Control—tumor, lymph nodes, metastasis
- NPO
Non-profit organization
- FOLFOX
Fluorouracil, leucovorin, oxaliplatin
- GEM
Gemcitabine
Author contributions
I was the primary surgeon for this surgery and the main author for this paper. Dr. Kawamoto proofread this paper. Any other authors participated in the operations or post-operative management.
Funding
None.
Availability of data and materials
Deidentified patient data will be available upon reasonable request to the corresponding author.
Declarations
Ethics approval and consent to participate
Our case report was approved by the ethics committee and the director of our hospital.
Consent for publication
The patient provided informed consent for the publication of this report.
Competing interests
The authors declare no competing interests with respect to this report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hoshino Y, Tsutsuyama M, Sugimoto H, et al. A case of aberrant pancreatic cancer in the small intestine with laparoscopic treatment. Jpn J Gastroenterol Surg. 2022;55:692–700. [Google Scholar]
- 2.Heinrich H. Ein Beitrag zur Histologie des sogen. Akzessorischen Pankreas. Virchows Arch path Anat. 1909;198:392–401. [Google Scholar]
- 3.UICC (Union for International cancer control). 8th edition. 2017.
- 4.Honma Y, Hirano H. Small bowel cancer, National Cancer Center and Rare cancer center websites. https://www.ncc.go.jp/jp/rcc/about/small_intestine_cancer. Accessed 4 Nov 2024.
- 5.de Castro Barbosa JJ, Dockerty MB, Waugh JM. Pancreatic heterotopia; review of the literature and report of 41 authenticated surgical cases, of which 25 were clinically significant. Surg Gynecol Obstet. 1946;82:527–42. [PubMed] [Google Scholar]
- 6.Mibayasi H, Ishikawa Y, Takekawa A, et al. Carcinoma originating from the heterotopic pancreas in the stomach, report of a case. Stomach Intest (Tokyo). 1983;18:267–72. [Google Scholar]
- 7.Takaku H, Nishimoto A, Yabusaki H. A case of tubular adenocarcinoma arising from ectopic pancreas in the stomach. Jpn Coll Surg. 2002;27:128–31. [Google Scholar]
- 8.Hirata K, Amano R, Kimura K, et al. A case of cancer of the ectopic pancreas developed in the duodenum. J Jpn Surg Assoc. 2013;74:1066–70. [Google Scholar]
- 9.Miyazaki T, Uenishi T, Kurashima Y, et al. A case of aberrant pancreatic cancer with an obstruction of the jejunum. J Abdom Emerg Med. 2017;37:1071–4. [Google Scholar]
- 10.Kinbara, November 25, 2022. Clinical practice guidelines for pancreatic cancer. 2022. pp 197–201.
- 11.Kinbara, December 20, 2016. General rules for the study of pancreatic cancer. 7th editors. 2016.
- 12.Fujiki T, Yamamoto K, Nishifuku K, Hutatsuki K, Nakajima T, et al. A case of heterotopic pancreatic carcinoma in the jejunum encountered in an abdominal operation. J Biliary Tract Pancreas. 1990;11(7):843–8. [Google Scholar]
- 13.Satoh T, Tabuchi S, Ino M, et al. A case of heterotopic pancreatic cancer in the jejunum. Jpn Surg Assoc. 1993;54:703–6. [Google Scholar]
- 14.Saigusa N, Tanaka T, Tsuchiya T, Yamaguchi T, Yanagisawa S, Kitakata Y. A case of small intestinal cancer thought to have arisen from ectopic pancreatic tissue. Ishosei sui sosiki kara hassei shitato kanngaerareta shouchougan no ichirei. Operation. 1995;49(5):715–7 (in Japanese). [Google Scholar]
- 15.Arao J, Fukui H, Hirayama D, et al. A case of aberrant pancreatic cancer in the jejunum. Hepatogastroenterology. 1999;46:504–7. [PubMed] [Google Scholar]
- 16.Uemura Y, Kobayashi K, Yoshida K, et al. A case of aberrant pancreas cancer in the jejunum. Jpn J Gastroenterol Surg. 2001;34:249–53. [Google Scholar]
- 17.Matsubara T, Tabara H. A case of adenocarcinoma of aberrant pancreas in the jejunum. J Jpn Surg Assoc. 2004;65:200–3. [Google Scholar]
- 18.Sato K, Maekawa T, Maekawa H, et al. A case of heterotopic pancreatic cancer in the jejunum. J Jpn Coll Surg. 2007;32:904–8. [Google Scholar]
- 19.Koh HC, Page B, Black C, et al. Ectopic pancreatic-type malignancy presenting in a Meckel’s diverticulum: a case report and review of the literature. World J Surg Oncol. 2009;7:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ono F, Hiraga M, Kudo K, et al. A case of adenocarcinoma of aberrant pancreas in the jejunum. Jpn Gastroenterol Surg. 2010;43:953–7. [Google Scholar]
- 21.Nagai S, Shinagawa Y, Morimatsu K, Tsutsumi N. A case of adenocarcinoma arising from aberrant pancreas in the jejunum. J Biliary Tract pancreas. 2014;35(8):781–5. [Google Scholar]
- 22.Okada K, Yagi S, Kinoshita H, et al. A case of adenocarcinoma arising from ectopic jejunum pancreas in which MRI aided the diagnosis. J Jpn Panc Soc. 2014;29:840–4. [Google Scholar]
- 23.Kurata Y, Arizono S, Hinoda T, et al. A case of adenocarcinoma of aberrant pancreas in the jejunum. Jpn J Clin Radiol. 2014;59(6):759–64. [Google Scholar]
- 24.Ogawa S, Fukushima M, Hobyung C, et al. A case of jejunal ectopic pancreatic cancer. Jpn J Gastroenterol Surg. 2015;112:70–7. [DOI] [PubMed] [Google Scholar]
- 25.Matsuyama T, Okuno K, Kamimoto M, et al. A case of adenocarcinoma of an aberrant pancreas in the jejunum with colonic stenosis. J Jpn Surg Assoc. 2015;76:2032–6. [Google Scholar]
- 26.Ito S, Takeda R, Kojima Y, et al. A case of adenocarcinoma of aberrant pancreas in the jejunum presenting with small bowel obstruction. J Jpn Surg Assoc. 2016;77:185–9. [Google Scholar]
- 27.Yogi N, Kaiho T, Yanagisawa S, et al. A case of jejunal ectopic pancreatic cancer with small bowel obstruction. Jpn J Cancer Chemother. 2017;44:1829–31. [PubMed] [Google Scholar]
- 28.Sakoda Y, Yokoyama Y, Miyamoto K, et al. Undifferentiated carcinoma in a Meckel’s diverticulum arising from ectopic pancreatic tissue. Jpn J Gastroenterol Surg. 2018;51:286–93. [Google Scholar]
- 29.Shigemasa Y, Maruyama K, Mori K, et al. A case of jejunal ectopic pancreatic cancer with intestinal perforation. Jpn J Cancer Chemother. 2019;46:2122–4. [PubMed] [Google Scholar]
- 30.Sakurai S, Ota Y, Matsushima S, et al. Adenocarcinoma arising in ectopic pancreas of Meckel’s diverticulum. Ann Gastroenterol Surg. 2019;52:465–74. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified patient data will be available upon reasonable request to the corresponding author.