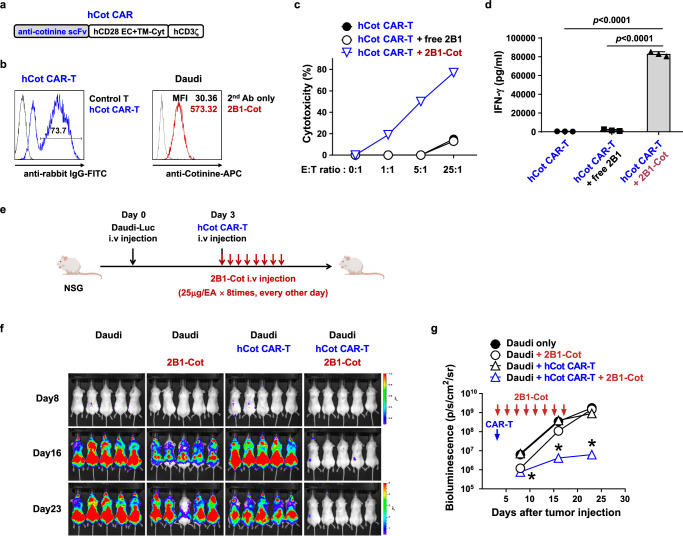
Fig. 5. Anti-tumor efficacy of anti-CD40 switchable CAR-T cells is recapitulated with an anti-human CD40 adapter and human Cot CAR-T cells in vivo.
a Schematic diagram of the hCot CAR construct. b Representative flow cytometry plot of Cot-CAR expression on human T cells 5 days after transduction (left); cotinine-labeled 2B1-Cκ (2B1-Cot) binding to Daudi cells (right). c Cytotoxicity of hCot CAR-T cells against Daudi cells preincubated with 2B1-Cκ (free 2B1) or 2B1-Cot. d IFN-γ production after co-culture of hCot CAR-T cells with Daudi cells preincubated with free 2B1 or 2B1-Cot for 24 h, measured in triplicate. Statistical significance was determined by an unpaired two-tailed t test. Results are representative of three independent experiments (c, d). e Experimental scheme for the treatment of human B-cell lymphoma xenografts with hCot CAR-T cells. NSG mice were injected i.v. with Daudi-Luc cells (5 × 105) on day 0 and hCot CAR-T cells (1 × 107) on day 3. From the day of CAR-T cell injection, 2B1-Cot (25 μg/head) was injected i.v. every other day for a total of 8 times. f, g Bioluminescence imaging of tumor burden at the indicated time points after Daudi-Luc cell injection (f). Bioluminescence intensity is calculated as the mean flux (p/s/cm2/sr) of a region of interest (ROI) in an individual mouse (g). Statistical significance between groups at each time point (n = 5) was determined by the nonparametric Kruskal-Wallis test. *: p = 0.0019 on day 8, p = 0.001 on day 16, and p = 0.0025 on day 23, compared to Daudi only group. Results are representative of at least two independent experiments. Data in (d and g) are presented as mean ± SEM. p: p-value. Source data are provided in the Source Data file.