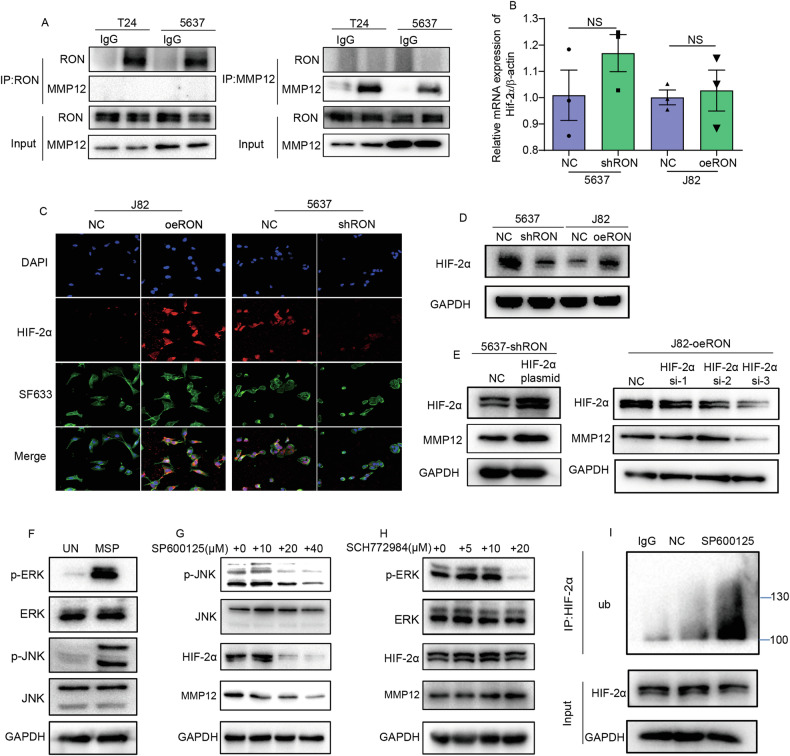
Fig. 4. RON modulates the expression of MMP12 via inhibiting ubiquitination of the transcription factor HIF-2α.
A Co-immunoprecipitation was employed to demonstrate the lack of direct interaction between RON and MMP12. B–D The mRNA expression levels of HIF-2α in 5637-shRON, 5637-NC, J82-oeRON, and J82-NC cells were quantified using quantitative real-time polymerase chain reaction (qRT-PCR), and confirmed by immunofluorescence staining and western blot analysis, respectively. E Transfection of 5637-shRON cells with a HIF-2α plasmid and J82-oeRON cells with HIF-2α-targeting siRNA was performed, followed by western blot analysis to assess the expression levels of HIF-2α and MMP12. F J82-oeRON cells were subjected to a 24-h period of starvation, followed by washing with ice-cold PBS to remove basal phosphorylation. Subsequently, the cells were stimulated with the RON-specific ligand MSP for 30 min, and western blot analysis was performed to assess the phosphorylation levels of ERK and JNK. G, H J82-oeRON cells were treated with varying concentrations of ERK inhibitors or JNK inhibitors for 24 h, after which the cells were harvested to evaluate the expression of HIF-2α and MMP12 by western blot. I J82-oeRON cells were subjected to treatment with or without 20 µM JNK inhibitors for a duration of 24 h, followed by the addition of 200 nM bortezomib 8 h prior to cell collection. Subsequently, the cells underwent immunoprecipitation using an anti-HIF-2α antibody, and the ubiquitination level of HIF-2α was assessed through immunoblotting with an anti-ubiquitin antibody. All experiments above were repeated three times (n = 3).