Abstract
Abstract
Objectives
To investigate the use of maintenance immunosuppressive treatments following liver transplantation and to compare their risk–benefit profiles in clinical practice.
Design
Retrospective multicentrer cohort study.
Setting
Four Italian regions (Lombardy, Veneto, Lazio, Sardinia).
Methods
Data were integrated from the national transplant information system and administrative claims data from four Italian regions. All adults who underwent incident liver transplantation between 2009 and 2019 were identified and categorised into two groups: cirrhosis or hepatocellular carcinoma (HCC). The trend of immunosuppressive treatment over years was analysed, and their effectiveness/safety profiles were compared using multivariate Cox models (HR; 95% CI).
Main outcome measures
Mortality, transplant reject/graft failure, incidence of severe infections, cancer, diabetes, major adverse cardiovascular events and lipid-modifying agents use.
Results
The study comprised 750 subjects in the cirrhosis cohort and 1159 in the HCC cohort. Over the study years, there was a decline in the use of cyclosporine-CsA, while combination therapy involving tacrolimus with other drugs increased compared with monotherapy. Overall, tacrolimus monotherapy use was slightly over 40% in both groups, followed by tacrolimus+mycophenolate (39.5%-cirrhosis; 30.6%-HCC) and tacrolimus+molecular target of rapamycin inhibitors (mTORi) (8.5%-cirrhosis; 13.3%-HCC). No significant differences emerged in risk–benefit profile of different tacrolimus-based therapies, except for a higher risk of mortality in cirrhosis subjects under tacrolimus monotherapy compared with tacrolimus+mycophenolate (HR: 2.07; 1.17 to 3.65).
Conclusions
The study highlights a shift over time in postliver transplant therapeutic patterns, favouring the use of tacrolimus in combination with mycophenolate or mTORi, rather than monotherapy. Moreover, a potential association between tacrolimus monotherapy and increased mortality in the cirrhosis cohort was identified. Further research is warranted to investigate these findings more deeply and to optimise treatment strategies for liver transplant recipients.
Keywords: Transplant medicine, Hepatology, EPIDEMIOLOGIC STUDIES, Drug Therapy, Drug Utilization
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Data were obtained from four Italian regions, which are representative of Northern, Central and Southern Italy; the large cohort enrolled and the record linkage of data coming from the National Transplant Centre reinforced the observed evidence.
One limitation of the study is that since it relies on administrative data, there is the possibility of clinical factors influencing the outcomes that we have not considered.
Immunosuppressive patterns were obtained from drugs reimbursed by the regional healthcare system; this means that medications prescribed in other regions or purchased privately were not considered.
Data on renal function and hepatocellular carcinoma recurrence were not available, even though the choice of some drugs (eg, molecular target of rapamycin inhibitors) may be linked to their antiproliferative effect and on the reduced risk of renal impairment.
Background
Liver transplantation (LT) is a life-saving procedure offered as the ultimate therapy for end-stage liver disease of any aetiology and for liver failure. Major advances have been achieved in the field of LT: the annual report of the European Liver Transplant Registry published in 20181 showed a marked improvement in survival rates over the years and 86% of 1-year survival rate between 2010 and 2014. Several factors, including advancements in surgical and anaesthesia techniques, perioperative care and better patients’ selection, have contributed to enhance outcomes of LT; among these factors, surely immunosuppression plays a key role in achieving such favourable outcomes. The growing effectiveness of immunosuppressive agents has led to a notable reduction in rejection rates and graft loss. On the other hand, the advantages of the increasing potency of immunosuppression have also resulted in prolonged exposure to these drugs and their associated undesirable collateral effect, creating a double-edged sword2; long-term adverse effects of immunosuppressant, including malignancies, opportunistic infections, metabolic disorders and organ toxicities, has emerged as a significant clinical issue.3 4
Current maintenance immunosuppressive regimens use multiple agents with different modes of action: calcineurin inhibitors (CNI), such as tacrolimus(TAC) and cyclosporine (CsA), remain still the cornerstone of immunosuppression for liver recipients and can be prescribed in combination with corticosteroids, mycophenolate mofetil(MMF), molecular target of rapamycin inhibitors (mTORi) (everolimus and sirolimus); the combination protocols permit the administration of drugs at reduced dosages without increasing the risk of allograft rejection and concurrently reducing the toxicity of individual agents.5 6 Although dosing guidelines are available for single medications, the overall approach to immunosuppression varies widely between transplant centres.7 8
Recently, an Italian working group composed of senior representatives of liver transplant centres published an article presenting evidence and consensus-based algorithms for guiding clinicians in selecting immunosuppressive strategies for different categories of adult liver transplant recipients.9 The authors divided the population in different categories based on the disease that led to transplant, underling that by dividing the population in specific categories based on primary disease and developing indications for each specific group they wanted to contribute to personalised and optimised immunosuppression; the work concluded that both for standard patients and critically ill (model for end-stage liver disease (MELD)>29, MELD 25–29 with renal dysfunction or other concurrent conditions) patients with cirrhosis immunosuppression should be based on TAC and CsA can be preferred as an alternative in particular in dysmetabolic patients; CNI monotherapy is not recommended and it is suggested the early introduction of MMF or mTORi for limiting CNI-related toxicity; in critically ill patients treatment should also be initiated at lower doses of TAC. Further, for all patients with hepatocellular carcinoma (HCC) authors reported advantages of minimising TAC and early adding of mTORi. Everolimus is particularly indicated in all patients at risk of renal dysfunction.
The present work was carried out as part of the CESIT project (Comparative evaluation of the Effectiveness and Safety of Immunosuppressive drugs in Transplanted patients), a multiregional pharmacovigilance initiative funded by the Italian Medicines Agency aiming to enhance the understanding of maintenance immunosuppression therapies administrated postsolid organ transplantation and to produce real-world evidence to inform prescribers on the use of these drugs in clinical practice. Hence, the aim of the study is to describe the patterns of usage and to compare the efficacy and safety profiles of the main maintenance immunosuppressive therapies prescribed in Italy taking into account the major indications for LT.
Materials and methods
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Study design
This study is a retrospective multicentre observational cohort study conducted across four Italian regions (Lombardy, Veneto, Lazio and Sardinia), which cover almost 20 million inhabitants, and where 47% of the Italian transplant activity takes place.
The study is based on data obtained from regional healthcare claims and the national transplant information system. Specifically, regional analytical datasets were created for incident patients who underwent liver transplant between 1 January 2009 (or first available date) and 31 December 2019, using information extracted from various claims data, including the hospital information system, pharmaceutical dispensation records, mortality information system and copayment exemption registry. Patients under 18 years of age, those who had prior history of transplantation and those with multiorgan transplants were not considered for inclusion. The data were standardised according to a common data model and shared using a distributed analysis tool called ‘TheShinISS’.10 Demographic and clinical characteristics of both donors and recipients, which were available nationwide, were linked; since clear information on subjects was not available, a semideterministic, intraregion linkage approach was adopted and a set of proxy information (eg, sex, organ type, year and month of birth, year and month of transplant, and transplant’s hospital) was used to link individuals with ‘pseudo-sensitive’ keys (eg, sex, organ type, year and month of birth, year and month of transplant, and transplant’s hospital).11 The study cohort was limited to patients residing in the considered regions who survived and had received at least one CNI immunosuppressive dispensation during the 30 days following their discharge (index period). Patients were then stratified based on the indication for liver transplant, cirrhosis or HCC, Milan criteria were used for selecting HCC patients for LT.12 Furthermore, patients were categorised according to the type of immunosuppressive therapy used during the index period. The initial differentiation was made between CNI-based therapies: TAC or CsA, and additional an distinction was made considering the combination with other immunosuppressor: MMF, mTORi, or none (monotherapy).
The use of therapies over time was analysed, and only those regimens that were still in use in the last years of the study were compared with each other in the analysis of outcomes.
In particular, each patient was followed up from 30 days after discharge until the occurrence of the study event, death, a maximum of 5 years, or the end of the study, whichever came first. The outcomes considered for the effectiveness analysis included mortality and transplant reject/graft failure. Data on transplant rejection registered in the national transplant information system was detected directly by clinicians following a finding of impairment of transplanted organ function due to histologically documented immunological causes. For safety analysis, the incidence of severe infections, cancer, diabetes, major adverse cardiovascular events (MACE) and lipid-modifying agents (LMAs) use were considered.
For each subcohort, multivariate Cox models (HR and 95% CI) were implemented to compare the efficacy-safety profile between the considered regimens. In the risk–benefit analysis, patients with a prior history of the outcome considered were excluded in order to include only patients at risk of experiencing it for the first time. An intention-to-treat analysis was conducted, with the follow-up period extending from day 31 after the discharge date up to a maximum of 5 years. The data were censored for death, end of study date, end of follow-up (5 years) or loss to follow-up. The covariates considered in the models were partly predefined based on factors potentially associated with exposure and outcome (ie, region, sex, age, discharge year, renal disease and dialysis, score MELD). Additionally, all factors found to be associated with the specific outcome, selected by a stepwise regression technique, were included.
Finally, in order to understand how often and when patients may have changed their treatment regimens over the course of the study, possible changes in therapy during follow-up were analysed, and time to switch was detected.
Results
Cohort selection and use of immunosuppressive therapies over time
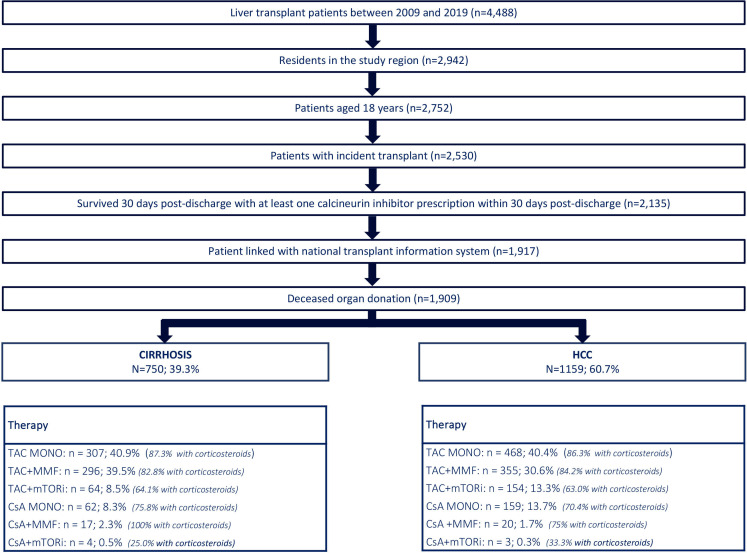
We identified a total of 4488 patients who underwent LT during the study period. Among them, 2942 individuals, representing 65.6% of the total, resided within the four regions under consideration. Within this subset, there were 2135 patients aged 18 years or older, who had undergone transplantation for the first time and survived for at least 30 days while using a CNI. Record linkage with the National Transplant Information System was successful, allowing identification of approximately 90% of patients using pseudoanonimous information (11). The 1909 dead donor liver transplants were stratified into 750 due to cirrhosis (39.3%) and 1159 for HCC (60.7%) (figure 1).
Figure 1. Study flowchart and immunosuppressive regimens in liver transplantation for patients with cirrhosis and HCC. HCC, hepatocellular carcinoma; mTORi, molecular target of rapamycin inhibitors. Note: TAC: Tacrolimus; MMF: Mycophenolate; mTORi: Mammalian target of rapamycin inhibitors; CsA: Cyclosporine; MONO: Monotherapy.
For both groups, the main immunosuppressor regimen was TAC in monotherapy with similar rates: 40.9% in cirrhosis and 40.4% in HCC (p value=0.8097). The use of TAC+MMF was more frequent in cirrhosis group than in HCC group (39.5% vs 30.6%; p value <0.0001), while the opposite was observed for TAC+mTORi with 8.5% and 13.3% (p-value=0.0014), respectively.
In addition, the use of corticosteroids in combination with TAC, either alone or with MMF, was high for both groups (>80%), while the combination TAC+mTORi+corticosteroid was less frequent (just over 60%).
Patient treated with CsA-based therapy, mainly in monotherapy, were 11% for cirrhosis and 15.3% for HCC.
The use of CsA has decreased over the years, suggesting the use of this therapy only for special cases; in 2014, CsA-based therapies were prescribed in 17.7% of patients with cirrhosis and in 27.7% of those with HCC; by the end of the study period in 2019, the use of these therapies was limited to 1.3% and 5.8% of patients, respectively (online supplemental figure 1A,B). Given the small number of CsA users and their decrease over time and considering that recent recommendations9 suggest limiting the use of CsA-based therapeutic regimens to specific cases, we decided to limit the analysis of outcomes to patients treated with TAC-based therapy only.
Characteristics of the cohort
Online supplemental table 1 shows demographic and clinical characteristics of these patients pertaining to the period before the transplant.
In the cirrhosis subcohort, with respect to TAC+MMF, the use of TAC-monotherapy was more frequent in Lombardy (69.1% vs 23.0%), affecting more patients who had longer hospitalisations (86.3% vs 77.0%) and a previous history of diabetes (47.2% vs 37.2%), while it was less frequent in those that had thyroid disorders (6.2% vs 10.8%) and anaemia (39.7% vs 53.4%). Instead, the use of TAC+mTORi was primarily observed in Veneto (46.9% vs 20.9%) and Lazio (45.3% vs 36.1%), in the younger population (18–49 years: 42.2% vs 31.8%), in the later years of the study (60.9% vs 35.1% between 2017 and 2019, consistent with online supplemental figure S1A), and among LMA users (25.0% vs 14.9%).
For HCC, while the considerations made for regions and comorbidities roughly remain the same (except for a greater use of TAC+MMF in Lazio), a higher use of TAC-monotherapy and TAC+mTORi is observed for older donors (>60 years: 60.9% and 66.9% vs 50.1%), while TAC+MMF is given more frequently to patients with MELD score greater than 25. Mean time of follow-up was 3.2 years (0.01–5.0) for the cirrhosis cohort and 2.9 years (0.02–5.0) for the HCC cohort; median time of follow-up was 3.5 years (1.8–5.00) and 2.9 years (1.3–5.0) respectively.
Effectiveness and safety analysis
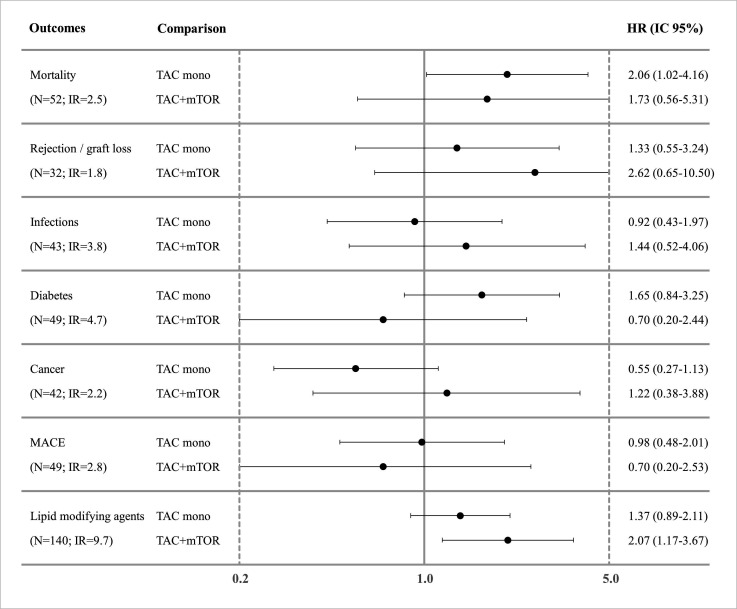
For cirrhosis, TAC-monotherapy was associated with a statistically significant higher risk of death (HR: 2.06; CI 95% 1.02 to 4.16) compared with TAC+MMF, but no statistically significant risk was observed for other outcomes in the study, including rejection (HR: 1.33; CI 95% 0.55 to 3.24) and incidence of MACE (HR:0.98; CI 95% 0.48 to 2.01). Moreover, TAC+mTORi seems to have the same risk–benefit profile as TAC+MMF, except for an increased risk of LMAs use (HR: 2.07; CI 95% 1.17 to 3.67) (figure 2).
Figure 2. HR for outcomes of interest respect by cirrhosis. Mortality: adjusted for region, sex, age, discharge year, renal disease and dialysis, score MELD, anaemia. Rejection/graft loss: adjusted for region, sex, age, discharge year, renal disease and dialysis, score MELD, BMI, diabetes, cardio-cerebrovascular diseases. Infections: adjusted for region, sex, age, discharge year, renal disease and dialysis, score MELD. Diabetes: adjusted for region, sex, age, discharge year, renal disease and dialysis, score MELD, thyroid disorders, anaemia, depression, BMI. Cancer: adjusted for region, sex, age, discharge year, renal disease and dialysis, score MELD, anticoagulants/antiplatelet. MACE: adjusted for region, sex, age, discharge year, renal disease and dialysis, score MELD, age (donor), diabetes, BMI, anticoagulants/antiplatelet. Use of LMAs: adjusted for region, sex, age, discharge year, renal disease and dialysis, score MELD, Charlson index, depression, diabetes. Note: TAC: Tacrolimus; BMI: body mass index; IR: incidence rate; LMAs: lipid-modifying agents; MACE: major adverse cardiovascular events; MELD: model for end-stage liver disease; mTORi:molecular target of rapamycin inhibitors.
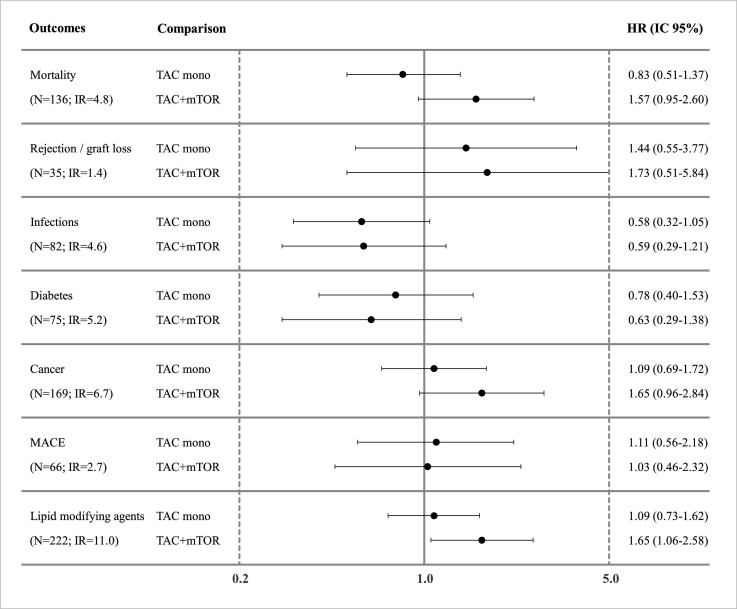
For HCC (figure 3), no significant difference was observed when comparing TAC-monotherapy versus TAC+MMF, except for a slight reduction, not statistically significant, in the risk of infection (HR: 0.58; CI 95% 0.32 to 1.05). Moreover, in this subcohort, a slight increase in the risk of death and cancer was estimated in patients using TAC+mTOR compared with TAC+MMF, even if these differences were not statistically significant (HR: 1.57; CI 95% 0.95 to 2.60 and HR: 1.65; CI 95% 0.96 to 2.84, respectively). Also, the use of mTORi was associated with an increased risk of incident LMAs use (HR:1.65; CI 95% 1.06 to 2.57).
Figure 3. HR for outcomes of interest respect by HCC. Mortality: adjusted for region, sex, age, discharge year, renal disease and dialysis, score MELD, age (donor), BMI (donor), cardio-cerebrovascular diseases, anaemia. Rejection/graft loss: adjusted for region, sex, age, discharge year, renal disease and dialysis, score MELD, diabetes, thyroid disorders. Infections: adjusted for region, sex, age, discharge year, renal disease and dialysis, score MELD, anaemia/antianemics, BMI (donor), anticoagulants/antiplatelet. Diabetes: adjusted for region, sex, age, discharge year, renal disease and dialysis, score MELD, diuretics. Cancer: adjusted for region, sex, age, discharge year, renal disease and dialysis, score MELD, diuretics, LMAs. MACE: adjusted for region, sex, age, discharge year, renal disease and dialysis, score MELD, Charlson index. Use of LMAs: adjusted for region, sex, age, discharge year, renal disease and dialysis, score MELD, cardio-cerebrovascular diseases. Note: TAC: Tacrolimus; BMI: body mass index; IR: incidence rate; LMAs: lipid-modifying agents; MACE: major adverse cardiovascular events; MELD: model for end-stage liver disease; mTORi:molecular target of rapamycin inhibitors.
Switches of immunosuppressive therapies during follow-up
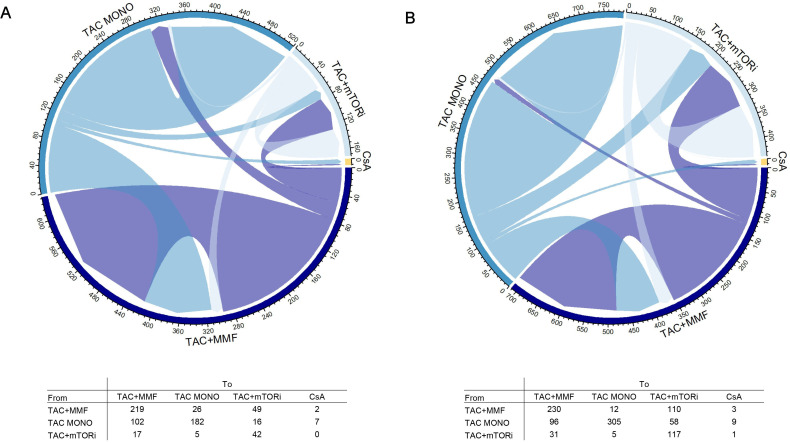
During follow-up (median time of 3.4 years), we observed several changes in immunosuppressive therapy over time for both subcohorts (figure 4A,B). Specifically, the most frequent therapy switch occurred from TAC-monotherapy to TAC+MMF in the cirrhosis group (102 individuals) and from TAC+MMF to TAC+mTORi in the HCC group (110 individuals). However, in this latter group, switching from TAC-monotherapy to TAC+MMF was also frequent (96 individuals). Overall, the rate of persistency in the initial therapy was good, with percentages ranging from 59% (182/307) for TAC-monotherapy in the cirrhosis group to 76% (117/154) for TAC+mTORi in the HCC group.
Figure 4. Chord diagram showing therapy switch during follow-up by cirrhosis (A) and HCC (B). HCC, hepatocellular carcinoma; mTORi, molecular target of rapamycin inhibitors. Note: TAC: Tacrolimus; MMF: Mycophenolate; mTORi: Mammalian target of rapamycin inhibitors; CsA: Cyclosporine; MONO: Monotherapy.
Online supplemental figure S2A and B shows the time to switch in the various therapy groups divided by the two subcohorts. It is observable that in both HCC and cirrhosis groups, the switch occurs earlier when switching from single to dual therapy, after 2.66 and 3.15 months, respectively, than in the other two groups.
Discussion
The present work was carried out to produce real-world evidence on the use and the efficacy-safety profile of post-transplant maintenance immunosuppressive therapies. To the best of our knowledge, this is the first observational study of its kind conducted in Europe.
First, the study highlighted that the use of cyclosporine, both as monotherapy and in combination with other drugs, has been abandoned in clinical practice over the years. This result is consistent with numerous evidence in literature indicating TAC as the CNI of choice13,15 and with consequent recommendations5 16. For example, a meta-analysis17 including 16 RCTs and 3813 patients showed that TAC significantly reduced the risks after LT of death, graft-loss, acute rejection and steroid-resistant rejection.
On the other hand, during the observation time, there has generally been a progressive increase in TAC use in combination therapy, and an increase in mTORi use in the HCC subcohort, these two facts also seem consistent with the prescribers’ consideration of the growing body of evidence supporting the use of combination therapy.
The strong variability in prescription choices among the different regions under study, as noted in online supplemental table 1, should be highlighted. This aspect had already been investigated previously,8 revealing significant variability between regions and over time in the choice of immunosuppressive therapies. This variability likely reflects the lack of consensus and guidelines on the treatment for these patients during the study period, as well as the lack of structured and specific diagnostic and therapeutic pathways for the treatment of post-LT recipients. Consequently, this may have led to different immunosuppressive strategies among various medical teams.
From the efficacy and safety analysis, it emerged that TAC-monotherapy was associated with an increased risk of mortality in the cirrhosis subcohort. This finding did not correspond with an increased risk of rejection, infections, or MACE. A possible explanation could be related to the nephrotoxic effects of CNI inhibitors, leading to renal dysfunction in these patients, but this clinical information could not be traced from the administrative data in our study. One main reason why an early introduction of MMF or mTORi is recommended is to allow the lowering of the TAC dose and the consequent sparing of renal function.9 Therefore, it would be interesting to integrate our results with data concerning kidney function and to investigate this outcome.
The increased risk of death found in the cirrhosis group does not arise in the HCC group on TAC monotherapy, even though guidelines advise against monotherapy in these patients as well. We can hypothesise that this result does not emerge from our analysis because the population with HCC, compared with the cirrhosis group, exhibited lower MELD scores across all therapy groups and a reduced presence of clinical history with renal failure or dialysis (online supplemental table 1). Hence, assuming that renal function plays an important role in determining mortality in this population, it is possible that the HCC subcohort was less susceptible to this issue.
It should be emphasised, however, that in addition to immunosuppressive therapy, several other factors may evolve over time and affect patient survival, some of which are only partially detectable using administrative data (eg, advancements in surgical techniques, improved recipient selection). This aspect limits our study’s ability to evaluate the exclusive role of immunosuppressive therapy on survival due to the concern of residual confounding. Notably, a 2023 report18 published by the National Transplant Centre (CNT) showed that for adult LT patients from 2000 to 2020, the 1-year post-transplant survival rate was 87.2%, while the 5-year survival rate was 75.8%. However, when examining the more recent period from 2014 to 2020, the 1-year survival rate increased to 89.5%, exceeding 90% in 2020—a more than 10 percentage point improvement compared with 2000. While it is possible that better management and selection of immunosuppressive therapies over the years contributed to this improvement, many other factors that we do not yet fully understand or measure could also have played a significant role.
As pointed out in the Results section, our analysis suggested an increased risk of mortality and cancer occurrence related to mTORi use in HCC group, although statistical significance is not reached in either case. These results may be due to a bias by indication, whereby patients using these drugs were at higher risk of developing de novo tumours or recurrence of HCC in the first place. Indeed, online supplemental table 1 shows that the mTORi users were older (42.5% vs 47.4%) and received the organ from older donors (50.1% vs 66.9%). Previous findings have established that immunosuppression plays a crucial role in de novo tumorigenesis and mTORi correlates with a lower incidence of de novo malignancies, because of their antiproliferative properties19,21; therefore, mTORi are recommended in patients at increased risk of developing tumours.22 23 Although the risks presented in our study were adjusted for measured characteristics, it is possible that additional factors, such as familiarity, risky behaviours (eg, being a smoker or not), or clinical parameter, may have influenced the choice of therapy.
Finally, in both cohorts, the ìmTORi use was associated with an increased risk of LMA utilisation; this result fits in with the well-known effect of these drugs of inducing alterations in lipid balance, often prompting clinicians to prescribe LMAs during mTORi therapy or before its initiation as a preventive measure.23 24
Based on the analysis of treatment changes over time, the study demonstrated a generally favourable therapy persistency rate (from 59% to 76%). However, it is noteworthy that switches from TAC-monotherapy to TAC+MMF within the cirrhosis group and from TAC+MMF to TAC+mTORi within the HCC group occurred, raising the need for further investigation into the potential impact of these switches on risk analysis. Moreover, the analysis of the time to switch (online supplemental figure S2A and B) suggests that the shift from TAC-monotherapy to dual therapy, occurring at an earlier phase, are proactive and linked to the need to set up a combination therapy with a lower risk of adverse effects, aiming to stabilise patients. On the contrary, given their occurrence later in the treatment course, the switches from TAC+MMF and TAC+mTORi are more likely to be reactive responses to side effects or issues that emerged after the initial treatment plan was established. These variations in the timing of switches contribute to the complexity of comparing the different switchers.
The main strengths of this study include the large cohort size, the availability of data on immunosuppressive dispensation from four regions representing Northern, Central, and Southern Italy, and the integration of both administrative data and information from the national transplant information system. To obtain this, the present project benefited from the use of ‘TheShinISS’ tool, which allowed aggregation of information from various regions and source of data. These aspects have proven to be particularly important, especially in light of the new privacy regulations25 and the legal challenges to data integration they introduced. Additionally, a rigorous methodology was used to investigate the relationship between immunosuppressive therapy and outcomes, adjusting for all relevant factors measured in the data sources considered, which resulted in association with both exposure and outcomes.
However, the study has some limitations. First, as noted above, it would be important to supplement our results with data on renal function that would allow investigation of this outcome in the two cohorts. In fact, this represents one of the primary issues of the present study, as renal failure is a significant parameter that can impact outcomes following LT.
With regard to the HCC cohort, given the administrative nature of the data, it was not possible to assess the incidence of HCC recurrence and its association with the different therapies. In fact, a recently published meta-analysis26 pointed out that the choice of mTORi may be especially linked to its antiproliferative effect, which, in addition to reducing the risk of new-onset cancers, also reduces the chance of disease recurrence. Since our data sources make it difficult to distinguish between HCC already existing at the time of transplantation and disease recurrence, we preferred not to investigate this aspect; however, future studies would be needed to evaluate this point.
Second, the administrative nature of the data requires taking into account the possibility of unobserved clinical factors influencing outcomes, the record linkage of data from the CNT and the large enrolled cohort help to strengthen the observed evidence, but it would be of particular interest to integrate the data with clinical information related to patients’ family history, aetiology of hepatic disease, tumour staging and severity.
Finally, as immunosuppressive pattern relies on drugs reimbursed by the national healthcare system, this approach may lead to some inaccuracies, due to prescriptions from outside the region or medications purchased privately. Additionally, there may be an overestimation of drug usage if patients claimed the drugs at the pharmacy but do not actually take it. Nevertheless, it is worth noting that the Italian National Health System offers comprehensive coverage of immunosuppressant drugs, which are rarely paid for by individual patients due to their high costs. Consequently, the proportion of patients purchasing these drugs privately can be considered negligible. Investigations have demonstrated the Italian health information system’s effectiveness in capturing chronically used medications, such as immunosuppressants’ post-transplant.27
Conclusions
The study highlights a shift over time in post-liver transplant therapeutic patterns, favouring the use of TAC in combination with MMF or mTORi, rather than monotherapy. Moreover, a potential association between TAC-monotherapy and increased mortality in the cirrhosis cohort was identified, although more detailed data would be necessary to evaluate the absolute impact of immunosuppressive therapy on survival and other outcomes.
These findings seem to suggest a gradual adoption of current guidelines, emphasising the importance of implementing treatment approaches geared towards minimising the adverse effects of CNI. However, there is a need for additional research to explore other outcomes, specifically renal function, in order to optimise treatment strategies for LT recipients.
supplementary material
Acknowledgements
This study was conducted in the context of the multiregional active pharmacovigilance CESIT project, funded by the Italian Medicines Agency. This retrospective study protocol was notified to the Ethical Committee of the Local Health Authority Roma 1, the reference ethical committee for the project’s coordinating centre (Department of Epidemiology of Lazio), according to the current national law.
Footnotes
Funding: This work was supported by the Italian Medicines Agency in the context of the multiregional pharmacovigilance project (AIFA 2012–2014: Comparative Effectiveness and Safety of Immunosuppressive Drugs in Transplant patients—CESIT project). Grant code: J85I2000009005 (CUP).
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-087373).
Provenance and peer review: Not commissioned; externally peer-reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and was approved by Comitato Etico Lazio 1(ordinance n. 1336, 5/11/2020). The study was approved by 'Comitato Etico Lazio 1' (ordinance n. 1336, 5/11/2020), the reference ethical committee for the project’s coordinating centre (Department of Epidemiology of Lazio, Lazio Regional Health Service, ASL Rome 1), according to the current national law. The informed consent could not be obtained since this retrospective study used exclusively data which are routinely gathered by the Italian Regions and National Transplant Center to inform policy decisions and to improve health public services. 'Comitato Etico Lazio 1' waived the requirement for informed consent. No participants below 16 years of age were considered. The study was conducted in accordance with relevant guidelines and regulations.
Data availability free text: The data that support the findings of this study are available from the Italian regions participating to CESIT study but restrictions apply to the availability of these data, which were used under license (as by third-party sources) for the current study, and so are not publicly available. However, data are available with permission of Italian regions, which are the data owner. The non-author contact information to which data requests may be sent is: project.cesit@gmail.com.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Collaborators: CESIT Study Group: Alessandro C. Rosa, Marco Finocchietti, Francesca R Poggi, Maria Lucia Marino, Arianna Bellini, Claudia Marino, Ursula Kirchmayer, Nera Agabiti, Marina Davoli, Antonio Addis, Valeria Belleudi (Department of Epidemiology, Lazio Regional Health Service); Marco Massari, Stefania Spila Alegiani (Pharmacoepidemiology Unit, National Centre for Drug Research and Evaluation, Istituto Superiore di Sanità, Rome); Lucia Masiero, Andrea Ricci, Bedeschi Gaia, Francesca Puoti, Vito Sparacino, Pamela Fiaschetti, Silvia Trapani, Alessandra Oliveti, Daniela Peritore, Massimo Cardillo (Italian National Transplant Center – Istituto Superiore di Sanità); Lorella Lombardozzi (Lazio Region); Silvia Pierobon, Eliana Ferroni, Maurizio Nordio, Manuel Zorzi (Veneto Region); Martina Zanforlini, Arianna Mazzone, Michele Ercolanoni, Giuseppe Piccolo, Andrea Angelo Nisic, Olivia Leoni (Lombardy Region); Stefano Ledda. Paolo Carta, Donatella Garau (Sardinia Region); Valentina Ientile, Luca L’Abbate (Messina University), Matilde Tanaglia, Gianluca Trifirò, Ugo Moretti (Verona University); Ersilia Lucenteforte (Pisa University).
Contributor Information
Arianna Bellini, Email: a.bellini@deplazio.it.
Marco Finocchietti, Email: m.finocchietti@deplazio.it.
Alessandro Cesare Rosa, Email: a.rosa@deplazio.it.
Lucia Masiero, Email: lucia.masiero@iss.it.
Silvia Trapani, Email: silvia.trapani@iss.it.
Massimo Cardillo, Email: massimo.cardillo@iss.it.
Marco Massari, Email: marco.massari@iss.it.
Stefania Spila Alegiani, Email: stefania.spila@iss.it.
Silvia Pierobon, Email: silvia.pierobon@azero.veneto.it.
Eliana Ferroni, Email: eliana.ferroni@azero.veneto.it.
Martina Zanforlini, Email: martina.zanforlini@ext.ariaspa.it.
Olivia Leoni, Email: Olivia_Leoni@regione.lombardia.it.
Stefano Ledda, Email: stledda@regione.sardegna.it.
Donatella Garau, Email: dgarau@regione.sardegna.it.
Marina Davoli, Email: m.davoli@deplazio.it.
Antonio Addis, Email: a.addis@deplazio.it.
Valeria Belleudi, Email: v.belleudi@deplazio.it.
CESIT Study Group:
Alessandro C Rosa, Marco Finocchietti, Francesca R Poggi, Maria Lucia Marino, Arianna Bellini, Claudia Marino, Ursula Kirchmayer, Nera Agabiti, Marina Davoli, Antonio Addis, Valeria Belleudi, Marco Massari, Stefania Spila Alegiani, Lucia Masiero, Andrea Ricci, Bedeschi Gaia, Francesca Puoti, Vito Sparacino, Pamela Fiaschetti, Silvia Trapani, Alessandra Oliveti, Daniela Peritore, Massimo Cardillo, Lorella Lombardozzi, Silvia Pierobon, Eliana Ferroni, Maurizio Nordio, Manuel Zorzi, Martina Zanforlini, Arianna Mazzone, Michele Ercolanoni, Giuseppe Piccolo, Andrea Angelo Nisic, Olivia Leoni, Stefano Ledda, Paolo Carta, Donatella Garau, Valentina Ientile, Luca L’Abbate, Matilde Tanaglia, Gianluca Trifirò, Ugo Moretti, and Ersilia Lucenteforte
Data availability statement
Data may be obtained from a third party and are not publicly available
References
- 1.Adam R, Karam V, Cailliez V, et al. 2018 Annual Report of the European Liver Transplant Registry (ELTR) - 50-year evolution of liver transplantation. Transpl Int. 2018;31:1293–317. doi: 10.1111/tri.13358. [DOI] [PubMed] [Google Scholar]
- 2.Di Maira T, Little EC, Berenguer M. Immunosuppression in liver transplant. Best Pract Res Clin Gastroenterol. 2020;46–47:S1521-6918(20)30016-0. doi: 10.1016/j.bpg.2020.101681. [DOI] [PubMed] [Google Scholar]
- 3.Åberg F, Gissler M, Karlsen TH, et al. Differences in long-term survival among liver transplant recipients and the general population: a population-based Nordic study. Hepatology. 2015;61:668–77. doi: 10.1002/hep.27538. [DOI] [PubMed] [Google Scholar]
- 4.Watt KDS, Pedersen RA, Kremers WK, et al. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant. 2010;10:1420–7. doi: 10.1111/j.1600-6143.2010.03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol. 2016;64:433–85. doi: 10.1016/j.jhep.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Panackel C, Mathew JF, Fawas N M, et al. Immunosuppressive Drugs in Liver Transplant: An Insight. J Clin Exp Hepatol. 2022;12:1557–71. doi: 10.1016/j.jceh.2022.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charlton M, Levitsky J, Aqel B, et al. International Liver Transplantation Society Consensus Statement on Immunosuppression in Liver Transplant Recipients. Transplantation. 2018;102:727–43. doi: 10.1097/TP.0000000000002147. [DOI] [PubMed] [Google Scholar]
- 8.Marino ML, Rosa AC, Finocchietti M, et al. Temporal and spatial variability of immunosuppressive therapies in transplant patients: An observational study in Italy. Front Transplant . 2022;1:1060621. doi: 10.3389/frtra.2022.1060621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cillo U, De Carlis L, Del Gaudio M, et al. Immunosuppressive regimens for adult liver transplant recipients in real-life practice: consensus recommendations from an Italian Working Group. Hepatol Int. 2020;14:930–43. doi: 10.1007/s12072-020-10091-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massari M, Alegiani SS, Cas R, et al. Istituto Superiore Di Sanità “TheShinISS”: Un Applicativo Open-Source per La Conduzione Di Analisi Distribuite in Studi Di Farmacoepidemiologia Di Tipo Multi-Database. 2020.
- 11.Belleudi V, Rosa AC, Finocchietti M, et al. An Italian multicentre distributed data research network to study the use, effectiveness, and safety of immunosuppressive drugs in transplant patients: Framework and perspectives of the CESIT project. Front Pharmacol. 2022;13:959267. doi: 10.3389/fphar.2022.959267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 13.O’Grady JG, Burroughs A, Hardy P, et al. Tacrolimus versus microemulsified ciclosporin in liver transplantation: the TMC randomised controlled trial. Lancet. 2002;360:1119–25. doi: 10.1016/s0140-6736(02)11196-2. [DOI] [PubMed] [Google Scholar]
- 14.González-Pinto IM, Rimola A, Margarit C, et al. Five-year follow-up of a trial comparing Tacrolimus and cyclosporine microemulsion in liver transplantation. Transplant Proc. 2005;37:1713–5. doi: 10.1016/j.transproceed.2005.03.128. [DOI] [PubMed] [Google Scholar]
- 15.Nelson J, Alvey N, Bowman L, et al. Consensus recommendations for use of maintenance immunosuppression in solid organ transplantation: Endorsed by the American College of Clinical Pharmacy. Am Soc Transplant, Int Soc Heart Lung Transplant Pharmacotherapy. 2022;42:599–633. doi: 10.1002/phar.2716. [DOI] [PubMed] [Google Scholar]
- 16.McAlister VC, Haddad E, Renouf E, et al. Cyclosporin versus tacrolimus as primary immunosuppressant after liver transplantation: a meta-analysis. Am J Transplant. 2006;6:1578–85. doi: 10.1111/j.1600-6143.2006.01360.x. [DOI] [PubMed] [Google Scholar]
- 17.Todeschini L, Cristin L, Martinino A, et al. The Role of mTOR Inhibitors after Liver Transplantation for Hepatocellular Carcinoma. Curr Oncol. 2023;30:5574–92. doi: 10.3390/curroncol30060421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centro Nazionale Trapianti Valutazione di Qualità dell’attività del trapianto di fegato 2000-2020. https://trapianti.sanita.it/statistiche/attivita/2023_D_QUALITA_ORGANI_FEGATO_00-20.pdf Available.
- 19.Jiménez-Romero C, Manrique A, Marqués E, et al. Switching to sirolimus monotherapy for de novo tumors after liver transplantation. A preliminary experience. Hepatogastroenterology. 2011;58:115–21. [PubMed] [Google Scholar]
- 20.Vivarelli M, Dazzi A, Cucchetti A, et al. Sirolimus in liver transplant recipients: a large single-center experience. Transplant Proc. 2010;42:2579–84. doi: 10.1016/j.transproceed.2010.04.045. [DOI] [PubMed] [Google Scholar]
- 21.Colmenero J, Tabrizian P, Bhangui P, et al. De Novo Malignancy After Liver Transplantation: Risk Assessment, Prevention, and Management-Guidelines From the ILTS-SETH Consensus Conference. Transplantation. 2022;106:e30–45. doi: 10.1097/TP.0000000000003998. [DOI] [PubMed] [Google Scholar]
- 22.De Simone P, Fagiuoli S, Cescon M, et al. Use of Everolimus in Liver Transplantation: Recommendations From a Working Group. Transplantation. 2017;101:239–51. doi: 10.1097/TP.0000000000001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klintmalm GB, Nashan B. The Role of mTOR Inhibitors in Liver Transplantation: Reviewing the Evidence. J Transplant. 2014;2014:845438. doi: 10.1155/2014/845438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh S, Watt KD. Long-term medical management of the liver transplant recipient: what the primary care physician needs to know. Mayo Clin Proc. 2012;87:779–90. doi: 10.1016/j.mayocp.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regolamento 2016/679-GDR del Parlamento Europeo e del Consiglio del. 2016.
- 26.Grigg SE, Sarri GL, Gow PJ, et al. Systematic review with meta-analysis: sirolimus- or everolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2019;49:1260–73. doi: 10.1111/apt.15253. [DOI] [PubMed] [Google Scholar]
- 27.Bennie M, Kurdi A, Mueller T. Encyclopedia of evidence in pharmaceutical public health and health services research in pharmacy. Cham: Springer; 2022. Using administrative data from public health and drug programs. [Google Scholar]