Fig. 1.
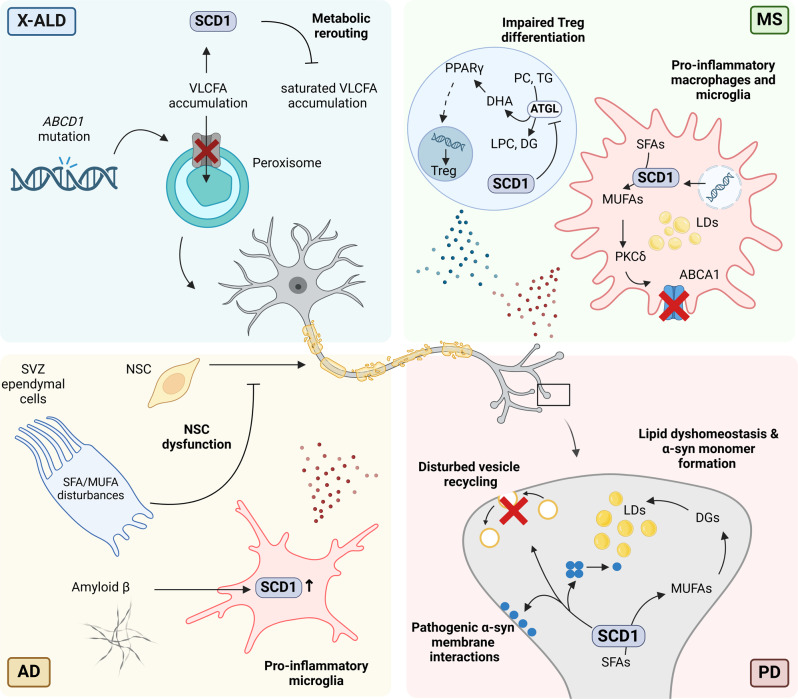
SCD1 is a driver of immune, glia, and neuronal physiology in neurodegenerative disorders such as X-ALD, MS, AD, and PD. In X-linked adrenoleukodystrophy (X-ALD, top left), SCD1 plays a crucial protective role by redirecting saturated to monounsaturated very long–chain fatty acids (VLCFAs), thereby mitigating VLCFA-induced lipotoxicity. However, contrary to X-ALD, heightened activity and abundance of SCD1 drive disease progression in multiple sclerosis (MS), Alzheimer’s disease (AD), and Parkinson’s disease (PD). In MS (top right), SCD1-produced monounsaturated FAs (MUFA) destabilize plasma membrane ATP-binding cassette transporter ABCA1 in a PKCδ-dependent manner, leading to intracellular accumulation of highly inflammatory free cholesterol in foamy myelin-containing macrophages and microglia. Moreover, SCD1 acts as a brake on Treg differentiation, exacerbating neuroinflammation by suppressing adipose triglyceride lipase (ATGL)-dependent release of docosahexaenoic acid (DHA) and subsequent peroxisome proliferator-activated receptor γ (PPARγ) activation. In AD (bottom left), disrupted saturated FAs (SFA)/MUFA ratios disrupt ependymal cell function in the subventricular zone (SVZ), impairing neural stem cell (NSCs) physiology (e.g. proliferative deficits), a hallmark of AD. Additionally, SCD1 exacerbates the formation of a disease-promoting microglial phenotype upon amyloid β engulfment. In PD (bottom right), SCD1 is implicated in α-synuclein (α-syn)-induced vesicle trafficking deficits and pathogenic interactions with membranes. Increased SCD1-generated oleic acid (OA) enhances ER-mediated α-syn toxicity, potentially through incorporation into diglycerides (DGs) and lipid droplets (LDs), while also promoting the formation of aggregation-prone α-syn monomers, thus heightening α-syn membrane association and neurotoxicity