Reaction optimisation.
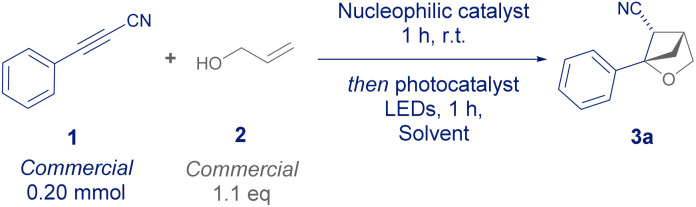
| ||||
|---|---|---|---|---|
| Entry | Solvent | Nucleophilic catalyst | Photocatalyst | Yielda (d.r.)b |
| 1 | Acetone | PBu3 (10 mol%) | TXO (15 mol%) | 26% (6 : 1) |
| 2 | Toluene | PBu3 (10 mol%) | TXO (15 mol%) | 52% (5 : 1) |
| 3 | DCM | PBu3(10 mol%) | TXO (15 mol%) | >99% (7 : 1) |
| 4 | DCM | PMe3 (10 mol%) | TXO (15 mol%) | 96% (7 : 1) |
| 5 | DCM | PPh3 (10 mol%) | TXO (15 mol%) | 12% (5 : 1) |
| 6 | DCM | NEt3 (10 mol%) | TXO (15 mol%) | n.d. |
| 7 | DCM | PBu3 (10 mol%) | [Ir cat] (2 mol%) | 40% (8 : 1) |
| 8 | DCM | PBu3 (10 mol%) | Ph2CO(15 mol%) | 43% (7 : 1) |
| 9 | DCM | PBu 3 (5 mol%) | TXO (5 mol%) | 86%c (8 : 1) |
| 10 | DCM | PBu3 (5 mol%) | — | <5% |
| 11 | DCM | — | TXO (5 mol%) | n.d. |
| 12d | DCM | PBu3 (5 mol%) | TXO (5 mol%) | n.d. |
1H NMR yield against internal standard.
Determined via integration of crude 1H NMR spectrum.
Isolated yield.
No irradiation. TXO = thioxanthen-9-one (370 nm irradiation); [Ir cat] = Ir(dFCF3ppy)2(dtbbpy)PF6 (456 nm irradiation); Ph2CO (370 nm irradiation).
