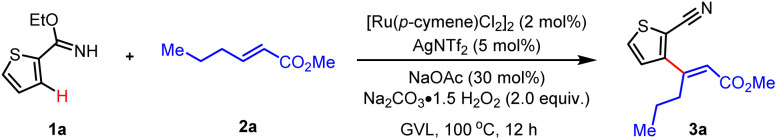
Oxidative Heck reaction of heterocycles with internal olefinsa.
| ||
|---|---|---|
| Entry | Variation of standard conditions | Yieldb (%) |
| 1 | Standard conditions | 76 |
| 2 | Pd(OAc)2, NiCl2 or [IrCp*Cl2]2 as the catalyst | n.r. |
| 3 | [RhCp*Cl2]2 as the catalyst | 73 |
| 4 | AgSbF6 instead of AgNTf2 | 65 |
| 5 | Without [Ru(p-cymene)Cl2]2 | n.r. |
| 6 | Without AgNTf2 | 27 |
| 7 | Without NaOAc | Trace |
| 8 | HOAc, PivOH instead of NaOAc | 30, 38 |
| 9 | NaOTFA, NaOPiv instead of NaOAc | 56, 67 |
| 10 | Without Na2CO3·1.5H2O2 | 24 |
| 11 | AgOAc, DTBP or Cu(OAc)2 as the oxidant | 68, trace, 66 |
| 12 | DCE, acetone, tBuOH, EA as the solvent | 72, 34, 53, 22 |
| 13 | 30 °C, 60 °C or 80 °C | Trace, trace, 46 |
| 14 | 1-AdCO2H (0.5 equiv.), EtOH | <10 |
Standard conditions: 1a (0.10 mmol), 2a (0.20 mmol), [Ru(p-cymene)Cl2]2 (2 mol%), AgNTf2 (5 mol%), NaOAc (30 mol%), Na2CO3·H2O2 (2.0 equiv.), GVL (1.0 mL), 100 °C, 12 h.
Isolated yield.
