Figure 3.
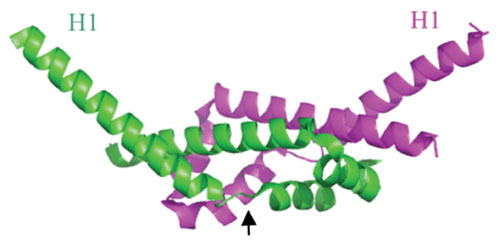
Ribbon diagram of the λ Gam protein dimer; chains A and B labeled green and magenta. It is an all α-helical protein with a dimerization domain (center region) and two protruding N-terminal helices (H1), sticking out at an angle of about 100° from each other. A proposed conformational change occurs upon binding of λ Gam to RecBCD, with the H1 helices rotating about 120° around the Gly-Ile-Pro hinge regions (denoted by arrow in the green subunit). The proposed conformation change places the H1 helices of each subunit into the ssDNA binding regions of RecB and RecCD, thus inhibiting binding of RecBCD to dsDNA ends. Structure generated by PyMol based on the coordinates described by Court et al. (83).
