Abstract
PURPOSE
To investigate the safety, tolerability, pharmacokinetics (PK), and preliminary antitumor activity of inavolisib, a potent and selective small-molecule inhibitor of p110α that promotes the degradation of mutated p110α, in combination with palbociclib and endocrine therapy (ET), in a phase I/Ib study in patients with PIK3CA-mutated, hormone receptor–positive/human epidermal growth factor receptor 2–negative locally advanced/metastatic breast cancer (ClinicalTrials.gov identifier: NCT03006172).
METHODS
Women ≥18 years of age received inavolisib, palbociclib, and letrozole (Inavo + Palbo + Letro arm) or fulvestrant (Inavo + Palbo + Fulv arm) until unacceptable toxicity or disease progression. The primary objective was to evaluate safety or tolerability.
RESULTS
Fifty-three patients were included, 33 in the Inavo + Palbo + Letro arm and 20 in the Inavo + Palbo + Fulv arm. Median duration of inavolisib treatment was 15.7 and 20.8 months (cutoff: March 27, 2023), respectively. Treatment-related adverse events (TRAEs) occurred in all patients; the most frequent were stomatitis, hyperglycemia, and diarrhea; grade ≥3 any TRAE rates were 87.9% and 85.0%; 6.1% and 10.0% discontinued any treatment due to TRAEs in the Inavo + Palbo + Letro and Inavo + Palbo + Fulv arms, respectively. No PK drug–drug interactions (DDIs) were observed among the study treatments when administered. Confirmed objective response rates were 52.0% and 40.0% in patients with measurable disease, and median progression-free survival was 23.3 and 35.0 months in the Inavo + Palbo + Letro and Inavo + Palbo + Fulv arms, respectively. Available paired pre- and on-treatment tumor tissue and circulating tumor DNA analyses confirmed the effects of study treatment on pharmacodynamic and pathophysiologic biomarkers of response.
CONCLUSION
Inavolisib plus palbociclib and ET demonstrated a manageable safety profile, lack of DDIs, and promising preliminary antitumor activity.
INTRODUCTION
Hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2–) breast cancer (BC) is the most common BC subtype.1 Initial treatment for advanced or metastatic disease includes endocrine therapy (ET) alone or in combination with targeted therapies; for example, cyclin-dependent kinase 4/6 inhibitor (CDK4/6i).2-4 Subsequent treatment options include ET alone or in combination with PI3K/AKT/mTOR pathway inhibitors.2-5 Clinical benefit with available therapies remain limited with the emergence of adaptive and acquired resistance.3,6 More effective treatment options that prevent or overcome resistance to available standard-of-care therapies are therefore critical.
CONTEXT
Key Objective
To investigate the safety, tolerability, pharmacokinetics, and preliminary antitumor activity of inavolisib plus standard of care (palbociclib plus either letrozole or fulvestrant) in patients with PIK3CA-mutated, hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2–) advanced breast cancer (BC) in a phase I/Ib dose-escalation study (ClinicalTrials.gov identifier: NCT03006172; GO39374).
Knowledge Generated
Inavolisib showed a manageable safety profile with good tolerability, lack of drug–drug interactions, and promising preliminary antitumor activity when combined with standard-of-care therapies.
Relevance
These encouraging data support the ongoing phase III INAVO120 study that evaluates inavolisib or placebo plus palbociclib and fulvestrant in patients with PIK3CA-mutated, HR+, HER2– advanced BC.
Dysregulated PI3K/AKT/mTOR pathway signaling has been implicated in both de novo and acquired endocrine resistance. Approximately 35%-40% of patients with HR+ BC have tumors that harbor mutations in phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), the gene encoding the alpha isoform catalytic subunit (p110α) of the PI3K complex.7-10 Furthermore, upregulation of growth factor signaling pathways, such as the PI3K/AKT/mTOR (PI3K) pathway, have been identified among a diverse array of aberrations implicated in ET and CDK4/6i resistance.11-14 Crosstalk among these connected signaling nodes underlies BC disease biology and response to therapy. In preclinical models, simultaneous inhibition of the estrogen receptor, CDK4/6, and PI3K pathways leads to synergistic effects on cell cycle arrest, apoptosis, and tumor regression in vitro and in vivo.15-19
SOLAR-1 established the combination of alpelisib (PI3Kα inhibitor) and fulvestrant as a standard of care for the treatment of PIK3CA-mutated, HR+, HER2– metastatic BC (mBC) that has progressed on or after an endocrine-based regimen. In clinical practice, however, implementation of this combination has been limited by its challenging safety profile that results in poor tolerability.20-22
Inavolisib is a potent and selective p110α inhibitor that promotes the degradation of mutated p110α. In biochemical assays, inavolisib was >300-fold more selective for p110α over the β, δ, and γ isoforms and demonstrated increased potency in tumor cells bearing mutated p110α over wild-type p110α.23,24 As inavolisib specifically promotes the degradation of mutated p110α, and not wild-type p110α, the endogenous p110α isoform and its downstream effectors that maintain homeostasis can be spared and may limit toxicity.23,24 Nonclinical studies have shown greater safety margins with inavolisib and greater, more durable target inhibition along with better in vivo efficacy.23,24 Furthermore, the combination of inavolisib, palbociclib, and fulvestrant in PIK3CA-mutated xenograft models has demonstrated greater antitumor efficacy compared with single-agent inavolisib and inavolisib in combination with either palbociclib or fulvestrant, supporting further exploration of inavolisib, palbociclib, and ET in clinical trials.23
GO39374 (ClinicalTrials.gov identifier: NCT03006172) is a first-in-human, phase I/Ib, open-label, dose-escalation study, evaluating the safety, tolerability, pharmacokinetics (PK), and preliminary antitumor activity of inavolisib as a single agent and in combination with ET and/or targeted therapies in PIK3CA-mutated locally advanced (LA)/mBC. Here, we report the clinical safety and tolerability, PK, preliminary antitumor activity, and analysis of pathway and pathophysiological biomarkers of response from treatment with inavolisib and palbociclib plus ET in patients with PIK3CA-mutated, HR+, HER2– LA/mBC.
METHODS
Study Design and Patients
The arm evaluating inavolisib in combination with palbociclib and letrozole (Inavo + Palbo + Letro arm) included a 3 + 3 dose-escalation design in stage I and stage II dose expansion; the arm evaluating inavolisib in combination with palbociclib and fulvestrant (Inavo + Palbo + Fulv arm) included a safety run-in to stage II dose-expansion.
Patients were postmenopausal women in the Inavo + Palbo + Letro arm and pre-, peri-, and postmenopausal women in the Inavo + Palbo + Fulv arm (pre- or perimenopausal women received gonadotropin-releasing or luteinizing hormone-releasing hormone agonist therapy ≥4 weeks before Cycle 1, Day 1 [C1D1] and on treatment), age ≥18 years, and had tumors that were known to harbor a PIK3CA mutation on the basis of the results from archival or fresh tumor tissue or circulating tumor DNA (ctDNA). Study-eligible mutations were H1047R/Y/L, E542K, E545K/D/G/A, Q546K/R/E/L, N345K, C420R, G1049R, R88Q, and M1043I. Patients had evaluable or measurable disease (per RECIST v1.1),25 Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1, life expectancy of ≥12 weeks, fasting glucose ≤140 mg/dL and glycosylated hemoglobin (HbA1c) <7%, and adequate hematologic and organ function within 14 days before initiation of study treatment, including absolute neutrophil count ≥1,500/μL. Key exclusion criteria included treatment with chemotherapy, immunotherapy, or biologic therapy as anticancer therapy within 3 weeks before initiation of study treatment, or ET within 2 weeks before initiation of study treatment; known and untreated, or active central nervous system metastases; type 1 or 2 diabetes requiring antihyperglycemic medication. For the stage I (dose-escalation) Inavo + Palbo + Letro arm, additional exclusion criteria included history of previous significant toxicity related to a CDK4/6i requiring discontinuation of treatment. For stage II (dose expansion), additional exclusion criteria included any previous treatment with a PI3K inhibitor or CDK4/6i; history of previous significant toxicity related to mTOR inhibitor requiring discontinuation of treatment; previous treatment with more than one cytotoxic chemotherapy regimen in the metastatic setting. The use of antihistaminic agents for rash and steroid mouthwash for stomatitis was allowed, including prophylactically. Treatment assignment (open-label) was conducted using an interactive voice or web-based response system.
Study Oversight
GO39374 was designed by the senior academic authors and representatives of the sponsor (Genentech, Inc, South San Francisco, CA). Data were collected by the sponsor and analyzed in collaboration with the senior academic authors, who vouched for the completeness and accuracy of the data and analyses, and for the fidelity of the study to the protocol. GO39374 was performed in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. Protocol approval was obtained from an independent ethics committee for each participating site. Every patient gave written informed consent. A study team comprised the safety monitoring committee and reviewed cumulative safety data periodically throughout the study conduct.
Study Procedures/Assessments
Patients received inavolisib at their assigned dose level (stage I: 3 mg, 6 mg, or 9 mg; stage II: 9 mg) orally (PO) once daily on D1-28 along with palbociclib (125 mg) PO once daily on D1-21, and letrozole (2.5 mg) PO once daily on D1-28 of each 28-day cycle or fulvestrant (500 mg) via intramuscular injection in the clinic on D1 and D15 of C1 and approximately every 4 weeks starting at C2D1, of each 28-day cycle.
PIK3CA tumor mutation status was determined from site-directed local polymerase chain reaction (PCR) or next-generation sequencing testing of tumor tissue or plasma-derived ctDNA or sponsor central testing of fresh or archival tumor tissue with the PCR-based cobas PIK3CA Mutation Test (F. Hoffmann-La Roche Ltd, Basel, Switzerland). Hormonal status and HER2 status were assessed locally per institutional clinical guidelines.
The extent of disease was assessed by computed tomography and other imaging modalities within 28 days of C1D1, approximately every 8 weeks, and at treatment discontinuation. Responses were assessed by the investigator according to RECIST v1.1;25 an objective response was confirmed by repeat assessments ≥4 weeks after initial documentation. Methods for immunohistochemistry (IHC) analysis of paired tumor tissue samples and analysis of PIK3CA mutation allele frequency (MAF) dynamics from ctDNA are described in Appendix 1 (online only).
Study End Points
The primary end point was safety and tolerability, assessed through summaries of adverse events (AEs), changes in laboratory test results, and changes in vital signs; all patients who received at least one dose of study treatment were included in the safety analyses. AEs occurring on or after treatment on D1 were summarized by mapped term per the Medical Dictionary for Regulatory Activities and National Cancer Institute Common Terminology Criteria for Adverse Events v4.0 toxicity grade. The dose-limiting toxicity (DLT) assessment window for stage I in the Inavo + Palbo + Letro arm and the safety run-in assessment period for the Inavo + Palbo + Fulv arm was C1D1-28.
Antitumor activity end points were confirmed objective response, duration of response, clinical benefit rate, and progression-free survival (PFS; definitions reported in Appendix 1).
Statistical Analysis
This study was intended to obtain preliminary safety, PK, antitumor activity, and pharmacodynamic information in the safety-evaluable population. Continuous variables were summarized using means, standard deviations, medians, and ranges. Categorical variables were summarized using counts and percentages. 95% CIs were calculated for the PFS end point using the Kaplan-Meier method. Box plots were used to summarize AUC and minimum plasma concentration observed (Cmin) for the PK analyses.
RESULTS
Study Population
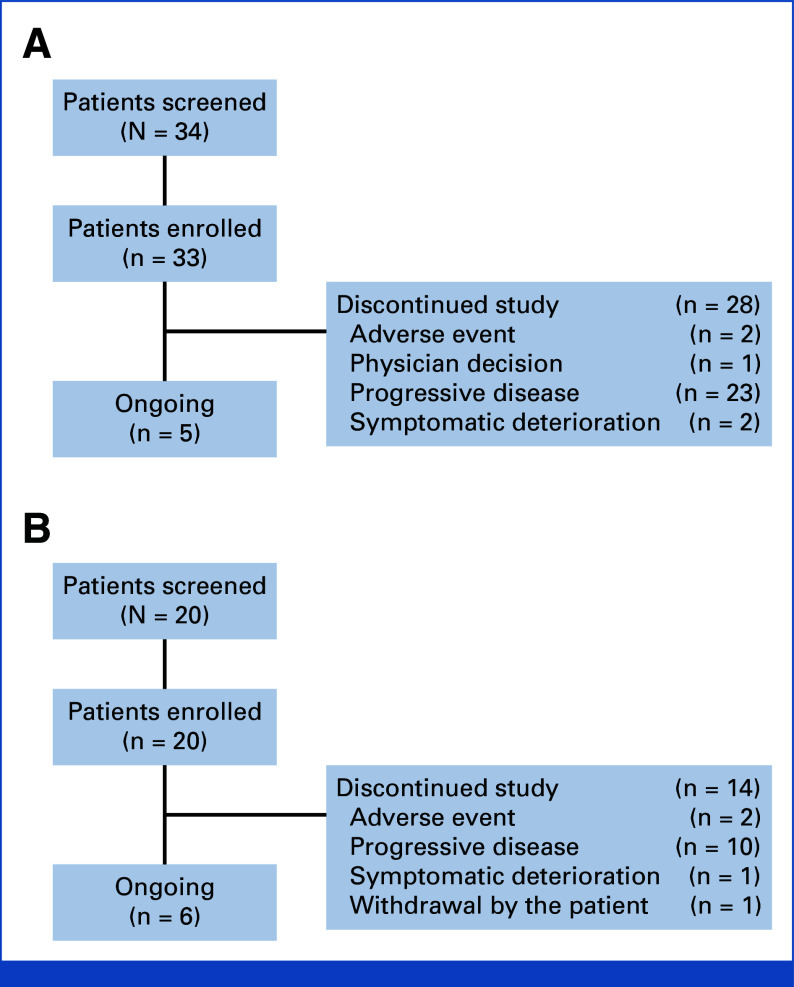
At data cutoff (March 27, 2023), 13 patients were enrolled in the stage I Inavo + Palbo + Letro arm (3 mg, n = 3; 6 mg, n = 3; 9 mg, n = 7). In stage II, 20 patients each were enrolled in the Inavo + Palbo + Letro and Inavo + Palbo + Fulv arms (Fig 1). In the Inavo + Palbo + Letro arm and Inavo + Palbo + Fulv arm, respectively, the median age was 57 and 55 years; 60.6% and 50.0% had an ECOG PS score of 0 at baseline; and most patients were White (78.8% and 50.0%). The median prior lines of systemic therapy in the metastatic setting were 2 (range, 0-4) and 1 (range, 0-4); 78.8% and 75.0% were previously treated with chemotherapy; 21.2% and 0% were previously treated with a CDK4/6i; 72.7% and 80.0% were previously treated with an aromatase inhibitor; and 42.4% and 15.0% were previously treated with the selective estrogen receptor antagonist and degrader fulvestrant; 54.5% and 30% presented with liver metastasis at time of study entry in the Inavo + Palbo + Letro and Inavo + Palbo + Fulv arms, respectively (Table 1).
FIG 1.
Flow diagram: (A) Inavo + Palbo + Letro arm and (B) Inavo + Palbo + Fulv arm. Fulv, fulvestrant; Inavo, inavolisib; Letro, letrozole; Palbo, palbociclib.
TABLE 1.
Baseline Demographics and Previous Anticancer Treatments (safety-evaluable population)
| Patient Demographic, Disease Characteristic, or Prior Treatment | Inavo + Palbo + Letro Arm (n = 33) | Inavo + Palbo + Fulv Arm (n = 20) |
|---|---|---|
| Age, years | ||
| Median | 57 | 55 |
| Range | 37-80 | 33-73 |
| Sex, No. (%) | ||
| Female | 33 (100) | 20 (100) |
| Race, No. (%) | ||
| Asian | 1 (3.0) | 0 |
| Black or African American | 1 (3.0) | 0 |
| White | 26 (78.8) | 10 (50.0) |
| Unknown | 5 (15.2) | 10 (50.0) |
| Ethnicity, No. (%) | ||
| Hispanic or Latino | 1 (3.0) | 1 (5.0) |
| Not Hispanic or Latino | 29 (87.9) | 8 (40.0) |
| No reported | 1 (3.0) | 4 (20.0) |
| Unknown | 2 (6.1) | 7 (35.0) |
| ECOG performance status | ||
| 0 | 20 (60.6) | 10 (50.0) |
| 1 | 13 (39.4) | 10 (50.0) |
| Weight, kg | ||
| Median | 66.0 | 64.5 |
| Range | 49.0-159.0 | 45.0-101.0 |
| BMI, kg/m2 | ||
| Median | 26.3 | 24.7 |
| Range | 17.6-50.8 | 19.2-38.0 |
| Prior lines of therapy in the metastatic setting, No. | ||
| Median | 2 | 1 |
| Range | 0-4 | 0-4 |
| Previous therapies, No. (%) | ||
| Chemotherapy | 26 (78.8) | 15 (75.0) |
| In the metastatic setting | 13 (39.4) | 4 (20.0) |
| CDK4/6ia | 7 (21.2) | 0 |
| In the metastatic setting | 7 (21.2) | 0 |
| Aromatase inhibitor | 24 (72.7) | 16 (80.0) |
| In the metastatic setting | 20 (60.6) | 8 (40.0) |
| SERD (all Fulv) | 14 (42.4) | 3 (15.0) |
| Liver metastases at enrollment, No. (%) | 18 (54.5) | 6 (30) |
Abbreviations: CDK4/6i, cyclin-dependent kinase 4/6 inhibitor; ECOG, Eastern Cooperative Oncology Group; Fulv, fulvestrant; Inavo, inavolisib; Letro, letrozole; Palbo, palbociclib; SERD, selective estrogen receptor antagonist and degrader.
Previous treatment with a CDK4/6i was an exclusion criterion for stage II.
The median inavolisib treatment durations were 15.7 (range, 1.3-66.5) months and 20.8 (range, 1.8-47.6) months in the Inavo + Palbo + Letro and Inavo + Palbo + Fulv arms, respectively. The median cumulative dose intensities of inavolisib were 95.3% and 90.5% in the Inavo + Palbo + Letro and Inavo + Palbo + Fulv arms, respectively. The median treatment durations and median cumulative dose intensities for palbociclib, letrozole, and fulvestrant are reported in Appendix Table A1.
Safety
No DLTs were reported in stage I of the Inavo + Palbo + Letro arm. All patients had at least one treatment-related AE (TRAE; Appendix Table A2), the most common (≥30% in either arm) of which were stomatitis (66.7% and 90%, grouped terms), neutropenia (81.8% and 85.0%), hyperglycemia (63.6% and 70.0%), diarrhea (48.5% and 55.0%), thrombocytopenia (48.5% and 50.0%), anemia (57.6% and 45.0%), nausea (45.5% and 30.0%), decreased appetite (36.4% and 30.0%), leukopenia (27.3% and 30.0%), asthenia (18.2% and 30.0%), alopecia (45.5% and 25.0%), and rash (36.4% and 15.0%, grouped terms) in the Inavo + Palbo + Letro and Inavo + Palbo + Fulv arms, respectively (Table 2). Any grade 3-4 TRAEs occurred in 87.9% and 85%, and serious TRAEs occurred in 15.2% and 0% of patients in the Inavo + Palbo + Letro and Inavo + Palbo + Fulv arms, respectively (Appendix Table A2). Common treatments for selected AEs included dexamethasone mouthwash for stomatitis, metformin for hyperglycemia, loperamide for diarrhea, and topical hydrocortisone for rash (Appendix Table A3). AEs leading to dose modification (interruption, reduction, or withdrawal) of any study treatment occurred in 93.9% and 100% of patients in the Inavo + Palbo + Letro and Inavo + Palbo + Fulv arms, respectively (Appendix Table A2). AEs leading to inavolisib dose reduction occurred in 18.2% and 30.0% of patients in the Inavo + Palbo + Letro and Inavo + Palbo + Fulv arms, respectively (Appendix Table A2). AEs leading to any treatment discontinuation occurred in 6.1% of patients in the Inavo + Palbo + Letro arm (related grade 3 hyperglycemia [3.0%, n = 1] and unrelated grade 3 cerebrovascular disorder [3.0%, n = 1]) and 10.0% of patients in the Inavo + Palbo + Fulv arm (related grade 2 asthenia [5.0%, n = 1] and related grade 2 diarrhea [5.0%, n = 1]). No deaths occurred in either arm (Appendix Table A2).
TABLE 2.
Most Common (≥30%) Treatment-Related Adverse Events in the Inavo + Palbo + Letro and Inavo + Palbo + Fulv Arms (safety-evaluable population)
| Patient | Inavo + Palbo + Letro Arm (n = 33), No. (%) | Inavo + Palbo + Fulv Arm (n = 20), No. (%) | ||
|---|---|---|---|---|
| All Grade | Grade ≥3 | All Grade | Grade ≥3 | |
| Stomatitis (grouped terms)a | 22 (66.7) | 1 (3) | 18 (90) | 2 (10) |
| Neutropenia | 27 (81.8) | 22 (66.7) | 17 (85.0) | 14 (70.0) |
| Hyperglycemia | 21 (63.6) | 7 (21.2) | 14 (70.0) | 3 (15.0) |
| Diarrhea | 16 (48.5) | 0 | 11 (55.0) | 1 (5.0) |
| Thrombocytopenia | 16 (48.5) | 2 (6.1) | 10 (50.0) | 4 (20.0) |
| Anemia | 19 (57.6) | 2 (6.1) | 9 (45.0) | 1 (5.0) |
| Nausea | 15 (45.5) | 0 | 6 (30.0) | 0 |
| Asthenia | 6 (18.2) | 0 | 6 (30.0) | 0 |
| Decreased appetite | 12 (36.4) | 0 | 6 (30.0) | 0 |
| Leukopenia | 9 (27.3) | 4 (12.1) | 6 (30.0) | 3 (15.0) |
| Alopecia | 15 (45.5) | 0 | 5 (25.0) | 0 |
| Rash (grouped terms)b | 12 (36.4) | 0 | 3 (15) | 0 |
Abbreviations: Fulv, fulvestrant; Inavo, inavolisib; Letro, letrozole; Palbo, palbociclib.
Stomatitis grouped terms: stomatitis, mucosal inflammation, mouth ulceration, and tongue ulceration.
Rash grouped terms: rash, rash maculopapular, dermatitis acneiform, and hand dermatitis.
Pharmacokinetics
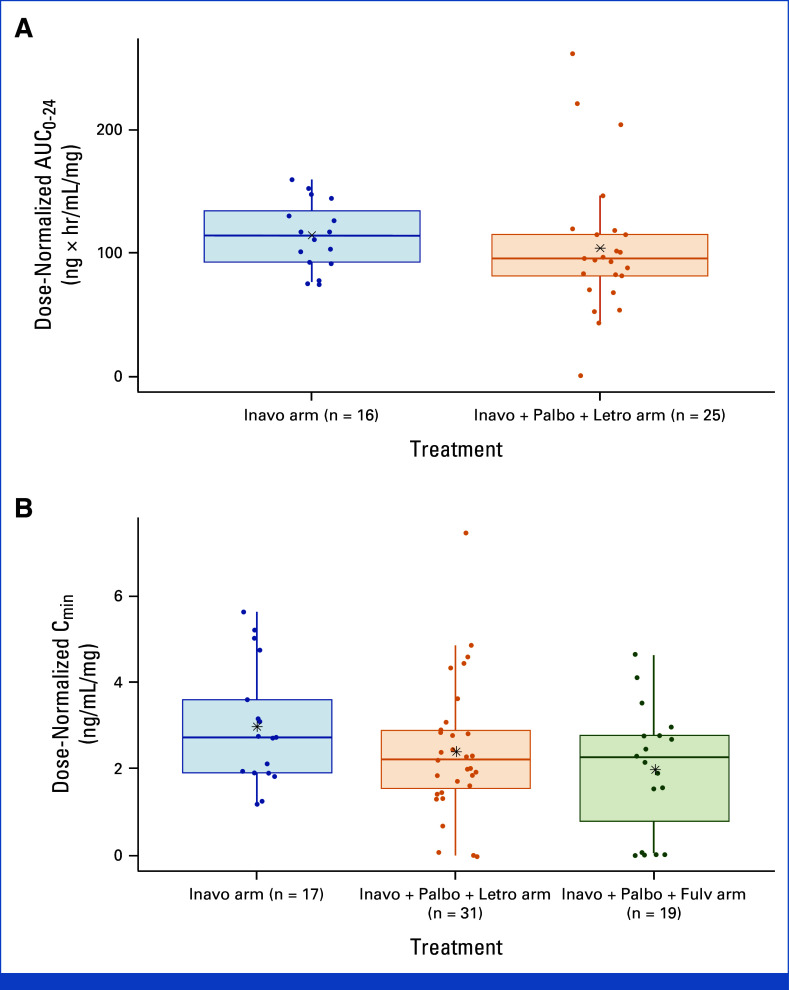
Results indicate that inavolisib dose-normalized steady state AUC from 0 to 24 hours after dosing (AUC0-24) and Cmin were similar between the inavolisib single-agent arm and the inavolisib-based combination arms, suggesting no impact of palbociclib, letrozole, or fulvestrant on inavolisib PK (Appendix Fig A1).
Palbociclib steady-state PK data from 22 patients in the Inavo + Palbo + Letro arm and 20 patients in the Inavo + Palbo + Fulv arm were evaluated. The steady-state Cmin geometric mean (geometric coefficient of variation [CV]%) of palbociclib in the Inavo + Palbo + Letro and Inavo + Palbo + Fulv arms was 48.3 ng/mL (107%) and 61 ng/mL (57.8%), respectively. Additional PK parameters are reported in Appendix Table A4. The PK of palbociclib in this study is comparable with historical single-agent PK of palbociclib,26 indicating no effect of inavolisib on the PK of palbociclib.
Letrozole steady-state PK data from 26 patients in the Inavo + Palbo + Letro arm were evaluated; the steady-state Cmin geometric mean (geometric CV%) was 0.334 μM (45%). These results show that the PK of letrozole is comparable with single-agent data reported in literature,27 suggesting no impact of inavolisib on letrozole PK.
Fulvestrant steady-state PK data from 16 patients in the Inavo + Palbo + Fulv arm were evaluated; the steady-state Cmin geometric mean (geometric CV%) was 13.3 ng/mL (36.8%) after several months of fulvestrant dosing. These results indicate that the PK of fulvestrant is comparable with historical single-agent data,28,29 suggesting no impact of inavolisib on fulvestrant PK.
Efficacy
Among patients with measurable disease at baseline, a best overall response of complete response or partial response was reported in 15 of 25 patients (60.0%) and in six of 15 patients (40.0%) in the Inavo + Palbo + Letro and Inavo + Palbo + Fulv arms, respectively. Among those patients, the confirmed objective response rates were 52.0% and 40.0%; the duration of response was 42.3 months (95% CI, 14.7 to not estimated) and 11.9 months (95% CI, 7.6 to 33.4) in the Inavo + Palbo + Letro and Inavo + Palbo + Fulv arms, respectively. Figure 2 reports the percentage change from baseline in sum of tumor diameters and the best overall response in patients with measurable disease.
FIG 2.
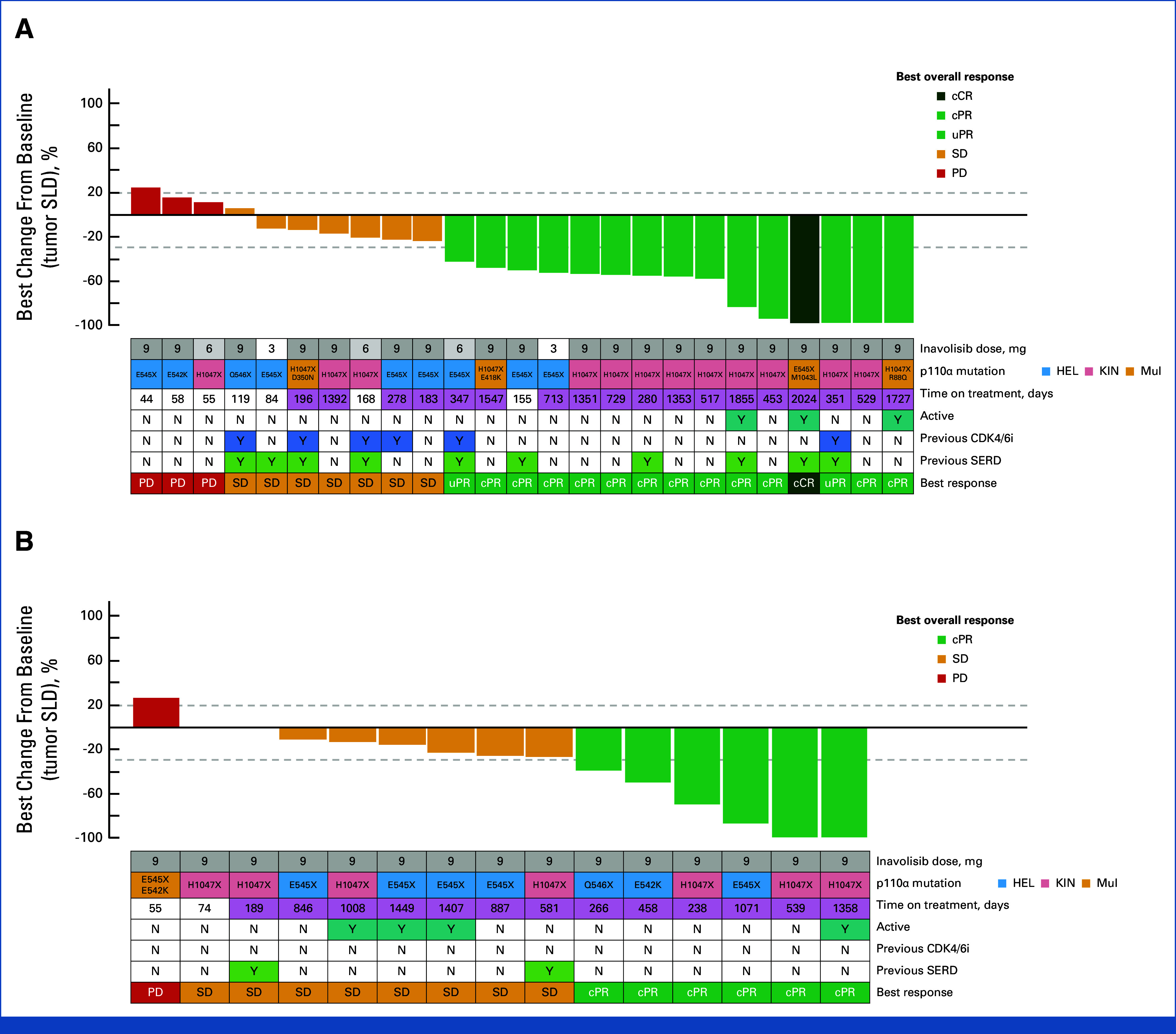
Best percentage change from baseline in tumor SLD in the (A) Inavo + Palbo + Letro arm and (B) Inavo + Palbo + Fulv arm (safety-evaluable population with measurable disease). X indicates the mutation result obtained from a PCR-based test that does not distinguish the exact amino acid change (ie, H1047X as opposed to H1047R or H1047Y, etc) and/or as provided by sites. Active is defined as on study treatment as of the clinical cutoff date. Time on treatment: purple shading indicates study treatment of at least 6 months. cCR, confirmed complete response; cPR, confirmed partial response; Fulv, fulvestrant; HEL, helical domain; Inavo, inavolisib; KIN, kinase domain; Letro, letrozole; mul, multiple mutations; Palbo, palbociclib; PCR, polymerase chain reaction; PD, progressive disease; SD, stable disease; SERD, selective estrogen receptor antagonist and degrader; SLD, sum of longest diameters; uPR, unconfirmed partial response.
In the overall patient population, the clinical benefit rate was 78.8% (26 of 33 patients) and 90.0% (18 of 20 patients) in the Inavo + Palbo + Letro and Inavo + Palbo + Fulv arms, respectively. The median PFS was 23.3 months (95% CI, 9.2 to 44.4) and 35.0 months (95% CI, 17.7 to not estimated) in the Inavo + Palbo + Letro and Inavo + Palbo + Fulv arms, respectively (Table 3).
TABLE 3.
Efficacy Summary
| Antitumor Activity End Point | Inavo + Palbo + Letro Arm (n = 33) | Inavo + Palbo + Fulv Arm (n = 20) |
|---|---|---|
| Patients with measurable disease | n = 25 | n = 15 |
| Best overall response, No. (%) | ||
| CR | 1 (4.0) | 0 |
| PRa | 14 (56.0) | 6 (40.0) |
| SD | 7 (28.0) | 8 (53.3) |
| PD | 3 (12.0) | 1 (6.7) |
| Best overall response rate, No. (%) | 15 (60.0) | 6 (40.0) |
| Confirmed objective response rate, No. (%) | 13 (52.0) | 6 (40.0) |
| All patients | n = 33 | n = 20 |
| Clinical benefit rate, No. (%) | 26 (78.8) | 18 (90.0) |
| PFS | ||
| Median, months (95% CI) | 23.3 (9.2 to 44.4) | 35.0 (17.7 to NE) |
Abbreviations: CR, complete response; Fulv, fulvestrant; Inavo, inavolisib; Letro, letrozole; NE, not estimable; Palbo, palbociclib; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
PR responses seen at all dose levels. CR response reported at the 9 mg dose level in the stage I Inavo + Palbo + Letro arm.
PIK3CA Mutation Spectrum and Analysis of Pharmacodynamic and Pathophysiologic Response Biomarkers
Reflective of the trial-eligible PIK3CA mutations (see Methods), patients' tumors were reported to harbor single PIK3CA mutations primarily localized to the helical (n = 21 patients; 40%) and kinase (n = 24 patients; 45%) domains; the remainder of patients' tumors harbored single PIK3CA mutations in the C2 domain (n = 2; 4%). Multiple PIK3CA mutations were reported in the tumors from six (11%) patients (Appendix Table A5).
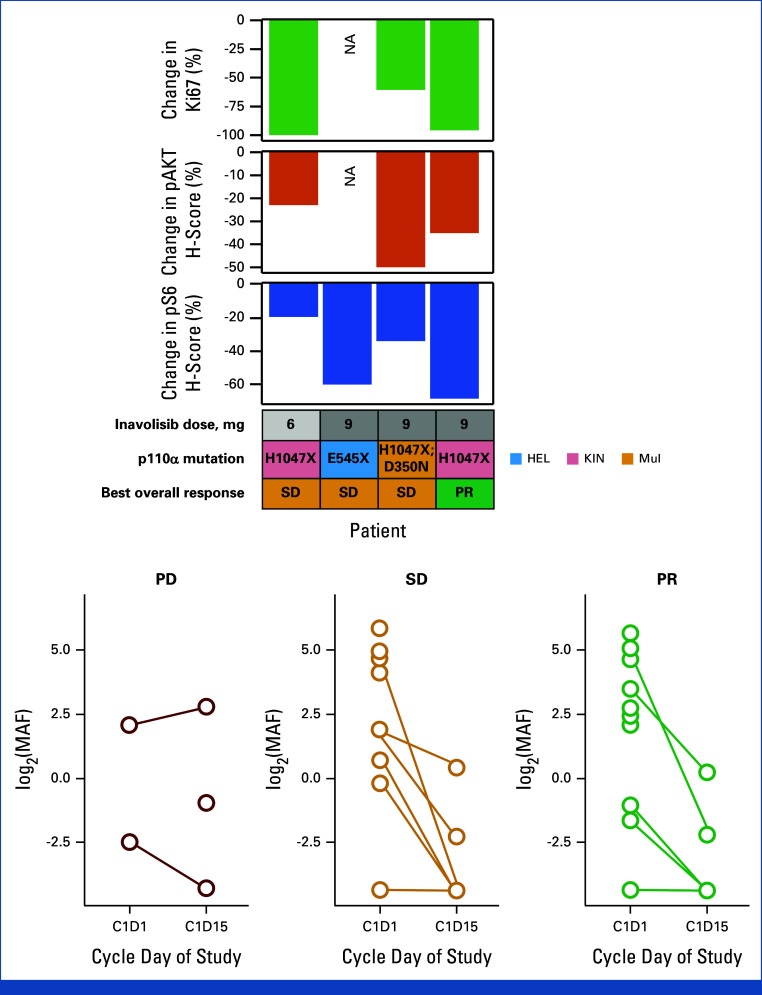
Four patients in the Inavo + Palbo + Letro arm with matched pretreatment (baseline) and on-treatment (C1D15) biopsy samples were evaluable for pharmacodynamic response analysis by IHC (no patients in the Inavo + Palbo + Fulv arm). PI3K pathway activity, on the basis of the expression of phosphorylated AKT (phosphorylated AKT; 36% average decrease [range, –22% to –49%]; n = 3) and phosphorylated S6 (phosphorylated S6; 46% average decrease [range, –20% to –69%]; n = 4), and proliferation as assessed by Ki67 nuclear staining (86% average decrease [range, –61% to –100%]; n = 3) were reduced (Fig 3A).
FIG 3.
Pharmacodynamic and pathophysiologic biomarker analyses. (A) % change from baseline to D15 of treatment in markers of proliferation (Ki67) and PI3K pathway activity (pAKT, pS6) as assessed by IHC (Inavo + Palbo + Letro arm; no paired IHC data available Inavo + Palbo + Fulv arm). Dose (mg), PIK3CA mutation type, and best overall response for each patient are shown. (B) MAF for PIK3CA as assessed by ctDNA at baseline (C1D1) and at 15 days on treatment (C1D15), grouped by best overall response. X indicates a mutation result obtained from a PCR-based test that does not distinguish the exact amino acid change (ie, H1047X as opposed to H1047R or H1047Y, etc) and/or as provided by sites. C, cycle; D, day; Fulv, fulvestrant; HEL, helical domain; IHC, immunohistochemistry; Inavo, inavolisib; KIN, kinase domain; Letro, letrozole; MAF, mutation allele frequency; mul, multiple mutations; NA, not available; pAKT, phosphorylated AKT; Palbo, palbociclib; PCR, polymerase chain reaction; PD, progressive disease; PR, partial response; pS6, phosphorylated S6; SD, stable disease.
To understand the pathophysiologic effects of study treatment, changes in PIK3CA MAF as detected between baseline and on-treatment (C1D15) ctDNA were analyzed. This analysis revealed a decrease in PIK3CA MAF between timepoints, reflective of tumor response to treatment in the majority of patients (5.68 average decrease [range, –34.77 to 2.54]; Fig 3B).
DISCUSSION
ET remains the cornerstone of treatment in both the early and metastatic settings for HR+ BC and has now evolved to include two-drug combinations with targeted therapies, including CDK4/6i and inhibitors of the PI3K pathway.2-4 However, crosstalk among the ER, CDK4/6, and PI3K pathways provides a scientific rationale for higher-order combinations targeting three or more signaling nodes to optimize clinical benefit and emulate results of similar combinations in HER2-positive BC, including the regimen of trastuzumab, pertuzumab, and docetaxel.15-19,30 A combinatorial drug screen of PIK3CA-mutated cancers found that combined CDK4/6–PI3K inhibition synergistically reduced tumor cell viability and that, in vivo, CDK4/6 plus PI3K inhibition can prevent or delay the time to CDK4/6i resistance.16,17 However, previous combinations with CDK4/6i that included continuous administration of alpelisib and other selective PI3K inhibitors have encountered significant toxicities, which prevented further clinical development.18,31
More recently, concurrent inhibition of the PI3K pathway in addition to ET and CDK4/6 inhibition has been investigated with novel agents given intermittently, including gedatolisib (an intravenously administered pan-class I isoform PI3K and mTOR inhibitor given weekly) and capivasertib (an oral small-molecule AKT inhibitor administered on a 4 days on/3 days off schedule).32,33 In a phase Ib study of gedatolisib with palbociclib and fulvestrant in patients with HR+, HER2– advanced BC previously treated with a CDK4/6i, the unconfirmed overall response rate was 63% (73% in patients with PIK3CA-mutated tumors), with a median PFS of 12.9 months.19,34 On the basis of the data from the CAPItello-291 trial, capivasertib in combination with fulvestrant was recently approved by the US Food and Drug Administration for the treatment of patients with HR+, HER2– LA/mBC harboring at least one PIK3CA/AKT1/PTEN alteration, following progression on at least one endocrine-based regimen in the metastatic setting or recurrence on or within 12 months of completing adjuvant therapy.5,35 Trials are underway to determine the clinical benefit of these PI3K pathway targeting agents in combination with CDK4/6i and ET (phase III VIKTORIA-1 trial and phase Ib/III CAPitello-292 trial).32,33
Data from the phase Ib study reported here demonstrate for the first time that continuous administration of inavolisib at its single-agent maximum tolerable dose of 9 mg once daily with palbociclib,36 and either letrozole or fulvestrant at standard doses and schedules, has a manageable safety profile that includes predominately low-grade stomatitis, hyperglycemia, and diarrhea, along with low rates of low-grade rash (all grade 1-2). Despite the rate of dose modification (interruption, reduction, or withdrawal), the median cumulative dose intensities of each treatment component remained high and, along with the long median treatment duration and overall low rates of treatment discontinuation due to AEs observed in this study, point to an overall well-tolerated combination treatment regimen. In addition, PK data demonstrate no drug–drug interactions (DDIs) among these agents, further supporting administration of this inavolisib-based cotargeting regimen at doses and schedules that maximize pathway inhibition.26-29 Albeit in small cohorts in this study, the translatability of the preclinical combination efficacy data is demonstrated by the promising and durable antitumor activity data for the combination of inavolisib, palbociclib, and ET with median PFS of 23.3 months in the Inavo + Palbo + Letro arm and 35.0 months in the Inavo + Palbo + Fulv arm. Though data were limited, the combination of inavolisib, palbociclib, and ETs decreased PI3K/AKT pathway biomarker expression and proliferation in paired tumor tissue samples, confirming both the mechanism of action of inavolisib and antitumor effects of this combination. Among patients with available paired pre- and on-treatment ctDNA samples and detectable PIK3CA mutation(s) at baseline, PIK3CA MAF decreased in all cases, although a comparison of PIK3CA MAF dynamics among patients who experienced progressive disease, stable disease, or partial response was limited by the overall cohort size and relatively few patients with best response of progressive disease. The PIK3CA MAF dynamics likely reflect the overall antitumor activity of the combination of inavolisib, palbociclib, and ETs and demonstrate the clinical utility of ctDNA to serve as an early surrogate marker of patient response to therapy.
These phase I/Ib study results provided the supportive evidence for the development of inavolisib in combination with CDK4/6i therapy and ET with the design of INAVO120 (ClinicalTrials.gov identifier: NCT04191499). INAVO120 is an ongoing phase III study that evaluates the combination of inavolisib, palbociclib, and fulvestrant in patients with PIK3CA-mutated, HR+, HER2–, LA/mBC whose disease progressed during treatment or within 12 months of completing adjuvant ET and who have not received previous systemic therapy for metastatic disease. INAVO120 demonstrated a statistically significant and clinically meaningful improvement in PFS, and manageable safety profile and tolerability.37
In conclusion, inavolisib with palbociclib plus ET demonstrated a manageable safety profile with good tolerability, lack of DDIs, and promising and durable preliminary antitumor activity in this phase I/Ib study. These data demonstrate the synergistic effect of targeting the ER, CDK4/6, and PI3K pathways in PIK3CA-mutated, HR+, HER2– LA/mBC and support the further evaluation of inavolisib and palbociclib plus fulvestrant in the phase III INAVO120 study.
ACKNOWLEDGMENT
The authors thank the patients and their families, participating study investigators, and clinical sites. Pfizer, Inc, provided palbociclib for this study. Research support in the form of third-party medical writing assistance, including the initial draft, was provided by Eleanor Porteous, MSc (Nucleus Global, an Inizio company), funded by F. Hoffmann-La Roche Ltd.
APPENDIX 1. SUPPLEMENTARY METHODS
Efficacy End Points
Antitumor activity end points were best overall response rate, defined as the proportion of patients with a complete or partial response, as determined by the investigator using RECIST v1.125; confirmed objective response rate, defined as the proportion of patients with a complete or partial response on two consecutive occasions ≥4 weeks apart, determined by the investigator using RECIST v1.125; duration of response, defined as the time from the first occurrence of a documented confirmed response of complete or partial response to disease progression, determined by the investigator using RECIST v1.1,25 or death, whichever occurred first; clinical benefit rate, defined as the percentage of patients achieving confirmed RECIST v1.125 defined complete response, partial response, and/or stable disease (noncomplete response/nonprogressive disease for patients with nonmeasurable disease at baseline) for ≥24 weeks; progression-free survival, defined as the time from the first study treatment (Day [D]1) to the first occurrence of disease progression, determined by the investigator using RECIST v1.1,25 or death, whichever occurred first.
Pharmacokinetics Methods
The pharmacokinetics (PK)-evaluable population consisted of all patients who received at least one dose of the study drug and had at least one evaluable postdose PK sample. PK parameters were derived from the plasma concentration-time profile of inavolisib following single-dose and multiple dose administration using NonCompartmental Analysis.
The systemic exposure of inavolisib in the presence of palbociclib and letrozole or palbociclib and fulvestrant was estimated using PK samples collected in the inavolisib plus palbociclib and letrozole (Inavo + Palbo + Letro) and inavolisib plus palbociclib and fulvestrant (Inavo + Palbo + Fulv) arms and compared with inavolisib single-agent PK from the inavolisib monotherapy arm.36 Given that inavolisib did not undergo extensive CYP-mediated metabolism in vitro or in the human mass balance study (unpublished data on file), drug-drug interactions with inavolisib and these concomitant medications were not anticipated. To assess the effect of 9 mg inavolisib on the PK of the other treatments in the inavolisib-based regimens, PK data of palbociclib, letrozole, and fulvestrant in the Inavo + Palbo + Letro arm and Inavo + Palbo + Fulv arm were compared with results from historical single-agent PK data.26-29
Immunohistochemical Analysis of Paired Tumor Tissue Samples
Paired formalin-fixed and paraffin-embedded tumor tissue samples collected from patients who consented to the optional paired biopsies before treatment (baseline) and during treatment (Cycle [C] 1 D15) underwent immunohistochemical analysis with antibodies against Ki67 (anti-Ki67 rabbit monoclonal primary antibody, clone 30-9, Ventana/Roche Diagnostics, Oro Valley, AZ) and phosphorylated AKT (Phospho-Akt [Ser473] [D9E] XP rabbit monoclonal antibody #4060, Cell Signaling Technology, Danvers, MA) and S6 (Phospho-S6 Ribosomal Protein [Ser235/236] [D57.2.2E] XP rabbit monoclonal antibody #4858, Cell Signaling Technology) at CellCarta (formerly HistoGeneX, in Belgium). Ki67 was scored as the total percentage of cells with nuclear staining in each sample. Cytoplasmic staining of phosphorylated AKT and phosphorylated S6 was scored by H-score methodology, where intensity is considered 0 for absent expression, 1+ for weak staining, 2+ for moderate staining, and 3+ for strong staining and H-score = (percentage of cells with staining absent × 0) + (percentage of 1+ cells × 1) + (percentage of 2+ cells × 2) + (percentage of 3+ cells × 3). For each patient, the percent change in each score was computed as 100 × a patient's (on-treatment – pretreatment)/pretreatment score and displayed as a bar plot to illustrate the change in staining/expression for each marker and sample pair.
Analysis of PIK3CA Mutation Allele Frequency Dynamics From Circulating Tumor DNA
Circulating tumor DNA (ctDNA) was isolated, as previously described,38 from plasma collected immediately before treatment (baseline, n = 27) and at C1D15 (n = 16) of treatment from study participants (paired baseline and C1D15 available for 14 of these) for sequencing with the FoundationACT comprehensive genomic profiling assay—a predecessor to FoundationOneLiquid CDx (F1LCDx)—or F1LCDx, in a Clinical Laboratory Improvement Amendments–certified, College of American Pathologists–accredited reference laboratory (Foundation Medicine, Inc, Cambridge, MA). Comprehensive details on these assay platforms, sequencing, and mutation calling methodologies were previously described.38 Mutant allele frequency (MAF) for PIK3CA is defined as the percent of mapped reads supporting the variant; cases where ctDNA was successfully sequenced but no PIK3CA mutation was detected are coded as MAF = 0. For patients with more than one PIK3CA single-nucleotide variation (SNV; includes both known pathogenic and unknown PIK3CA mutations), the sum of the MAFs of each SNV is used.
FIG A1.
Inavo dose normalized steady state plasma (A) AUC0-24 and (B) Cmin following multiple doses of Inavo administered as a single agent or in the presence of concomitant medications. AUC0-24, area under the concentration-time curve from 0 to 24 hours after dosing; Cmin, minimum plasma concentration observed; Fulv, fulvestrant; Inavo, inavolisib; Letro, letrozole; Palbo, palbociclib.
TABLE A1.
Median Treatment Durations and Median Cumulative Dose Intensities (safety-evaluable population)
| Treatment or Dose | Inavo + Palbo + Letro Arm (n = 33) | Inavo + Palbo + Fulv Arm (n = 20) |
|---|---|---|
| Treatment duration, months (range) | ||
| Inavo | 15.7 (1.3-66.5) | 20.8 (1.8-47.6) |
| Palbo | 15.7 (1.4-66.3) | 20.8 (1.8-47.6) |
| Letro | 17.0 (1.4-66.5) | — |
| Fulv | — | 20.2 (0.9-47.4) |
| Median cumulative dose intensities,a % (range) | ||
| Inavo | 95.3 (22.7-238.6) | 90.5 (43.2-99.8) |
| Palbo | 81.3 (43.6-101.0) | 80.6 (48.3-99.4) |
| Letro | 98.0 (76.5-100.0) | — |
| Fulv | — | 95.4 (30.8-225.6) |
Abbreviations: Fulv, fulvestrant; Inavo, inavolisib; Letro, letrozole; Palbo, palbociclib.
The cumulative dose intensity is the proportion of the total dose administered divided by the total dose planned to be administered. Some cumulative dose intensities for Inavo may be above 100% due to intrapatient dose escalation in stage I.
TABLE A2.
Safety Summary (safety-evaluable population)
| Patients | Inavo + Palbo + Letro Arm (n = 33), No. (%) | Inavo + Palbo + Fulv Arm (n = 20), No. (%) |
|---|---|---|
| Patients with at least one AE | 33 (100) | 20 (100) |
| Related to any study treatment | 33 (100) | 20 (100) |
| Related to Inavo | 33 (100) | 20 (100) |
| Patients with at least one grade 3-4 AE | 30 (90.9) | 17 (85.0) |
| Related to any study treatment | 29 (87.9) | 17 (85.0) |
| Related to Inavo | 19 (57.6) | 6 (30.0) |
| Patients with at least one serious AE | 14 (42.4) | 6 (30.0) |
| Related to any study treatment | 5 (15.2) | 0 |
| Related to Inavo | 4 (12.1)a | 0 |
| AE leading to dose modification (interruption, reduction, or withdrawal) of any treatment | 31 (93.9) | 20 (100) |
| AE leading to discontinuation of any treatment | 2 (6.1) | 2 (10.0) |
| Deaths | 0 | 0 |
Abbreviations: AE, adverse event; Fulv, fulvestrant; Inavo, inavolisib; Letro, letrozole; Palbo, palbociclib.
Grade 3 cellulitis, grade 3 wound cellulitis, grade 2 confusional state, and grade 2 osteomyelitis.
TABLE A3.
Treatment of Selected AEs (hyperglycemia, stomatitis, diarrhea, and rash) Administered in at Least Two Patients in Either Arm for Targeted Events
| AE Concomitant Treatment Received, Patients | Inavo + Palbo + Letro Arm (n = 33), No. (%) | Inavo + Palbo + Fulv Arm (n = 20), No. (%) |
|---|---|---|
| Hyperglycemia | 12 (36.4) | 11 (55.0) |
| Metformin | 12 (36.4) | 11 (55.0) |
| Sitagliptin | 5 (15.2) | 2 (10.0) |
| Empagliflozin | 5 (15.2) | 1 (5.0) |
| Dapagliflozin | 2 (6.1) | 1 (5.0) |
| Stomatitis | 19 (57.6) | 14 (70.0) |
| Dexamethasone | 14 (42.4) | 13 (65.0) |
| Lidocaine | 6 (18.2) | 4 (20.0) |
| Chlorhexidine | 4 (12.1) | 2 (10.0) |
| Triamcinolone | 4 (12.1) | 1 (5.0) |
| Clobetasol | 3 (9.1) | 2 (10.0) |
| Sodium chloride | 0 | 3 (15.0) |
| Clobetasol propionate | 2 (6.1) | 1 (5.0) |
| Glucose oxidase, lactoferrin, lactoperoxidase, lysozyme | 2 (6.1) | 1 (5.0) |
| Sodium bicarbonate | 2 (6.1) | 1 (5.0) |
| Benzocaine | 2 (6.1) | 0 |
| Clotrimazole | 2 (6.1) | 0 |
| Metamizole | 2 (6.1) | 0 |
| Stomatologic preparations | 2 (6.1) | 0 |
| Lidocaine, nystatin, water | 0 | 2 (10.0) |
| Diarrhea | 10 (30.3) | 6 (30.0) |
| Loperamide | 9 (27.3) | 3 (15.0) |
| Saccharomyces boulardii | 0 | 2 (10.0) |
| Rash | 8 (24.2) | 2 (10.0) |
| Hydrocortisone | 2 (6.1) | 0 |
| Sulfadiazine silver | 2 (6.1) | 0 |
| Bilastine | 2 (6.1) | 0 |
| Fluocinonide | 2 (6.1) | 0 |
Abbreviations: AE, adverse event; Fulv, fulvestrant; Inavo, inavolisib; Letro, letrozole; Palbo, palbociclib.
TABLE A4.
Palbo (125 mg PO once daily) Steady-State PK Parameter Summary
| PK Parameter | Study GO39374a | Palbo FDA Clinical Pharmacology Review (NDA 20,7103 review)b,c | ||
|---|---|---|---|---|
| Inavo + Palbo + Letro Arm (n = 22) | Inavo + Palbo + Fulv Arm (n = 20) | Study 1001 (n = 13) | Study 1003 (n = 12) | |
| Geometric mean Cmax, ng/mL (geometric CV%) | 90.7 (60) | — | 94.9 (48) | 116 (28) |
| Median Tmax, hours (range) | 8.0 (0-8) | — | 4.2 (2.0-9.8) | 7.9 (2.2-8.2) |
| Geometric mean AUC0-24, ng × h/mL (geometric CV%) | 1,650 (61) | — | 1,633 (59) | 1,982 (29) |
| Geometric mean Cmin, ng/mL (geometric CV%) | 48.3 (107) | 61.0 (58) | 47.0 (49) | 60.8 (42) |
Abbreviations: AUC0-24, area under the concentration–time curve from 0 to 24 hours after dosing; Cmax, maximum plasma concentration observed; Cmin, minimum plasma concentration observed; CV, coefficient of variation; FDA, US Food and Drug Administration; Fulv, fulvestrant; Inavo, inavolisib; Letro, letrozole; NDA, New Drug Application; Palbo, palbociclib; PK, pharmacokinetics; PO, orally; Tmax, time to maximum concentration.
Commercial freebase formulation of Palbo administered with a meal.
Isethionate capsule formulation of Palbo administered after an overnight fast.
Commercial freebase formulation administered with food has comparable exposure as the isethionate salt formulation on the basis of historical single-agent data of Palbo (NDA 20,7103 review).
TABLE A5.
PIK3CA Mutations Reported for Patients Enrolled in the Inavo + Palbo + Letro and Inavo + Palbo + Fulv Arms
| PIK3CA Mutation(s) Observed | Total in Category | PIK3CA Mutation Category | Patients With Tumors Harboring the Indicated PIK3CA Mutation, No. |
|---|---|---|---|
| E545X | Single helical: 40% | Helical | 14 |
| E542K | Helical | 4 | |
| Q546X | Helical | 3 | |
| H1047X | Single kinase: 45% | Kinase | 24 |
| C420R | Single C2 domain: 4% | C2 domain | 1 |
| N345K | C2 domain | 1 | |
| E545X; E542K | Multiple: 11% | Helical; helical | 1 |
| E545X; M1043L | Helical; kinase | 1 | |
| H1047X; D350N | Kinase; C2 domain | 1 | |
| H1047X; E418K | Kinase; C2 domain | 1 | |
| H1047X; P539R | Kinase; helical | 1 | |
| H1047X; R88Q | Kinase; other | 1 |
NOTE. X indicates the mutation result obtained from a PCR-based test that does not distinguish the exact amino acid change (ie, H1047X as opposed to H1047R or H1047Y, etc) and/or as provided directly by sites.
Abbreviations: Fulv, fulvestrant; Inavo, inavolisib; Letro, letrozole; Palbo, palbociclib; PCR, polymerase chain reaction.
Komal L. Jhaveri
Consulting or Advisory Role: Novartis, Pfizer, AstraZeneca, Jounce Therapeutics, Synthon, Intellisphere, Bristol Myers Squibb, Genentech, AbbVie, Lilly, BluePrint Medicines, Seagen, Daiichi Sankyo, Biotheranostics, Sun Pharma Advanced Research Company, Taiho Oncology, Sanofi, Gilead Sciences, Scorpion Therapeutics
Research Funding: Novartis (Inst), Genentech (Inst), Debiopharm Group (Inst), ADC Therapeutics (Inst), Pfizer (Inst), Novita Pharmaceuticals (Inst), Clovis Oncology (Inst), Lilly (Inst), Zymeworks (Inst), Immunomedics (Inst), Puma Biotechnology (Inst), VelosBio/Merck (Inst), AstraZeneca (Inst), Context Therapeutics (Inst), Scorpion Therapeutics (Inst), Blueprint Medicines (Inst)
Travel, Accommodations, Expenses: Taiho Pharmaceutical, Jounce Therapeutics, Pfizer, AstraZeneca, Intellisphere, Lilly, Gilead Sciences, Genentech/Roche
Melissa K. Accordino
Honoraria: Incrowd
Research Funding: Novartis (Inst), Genentech (Inst), Roche (Inst)
Other Relationship: Disney
Philippe L. Bedard
Research Funding: Bristol Myers Squibb (Inst), Sanofi (Inst), AstraZeneca (Inst), Genentech/Roche (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Merck (Inst), Seagen (Inst), Lilly (Inst), Amgen (Inst), Bicara Therapeutics (Inst), Zymeworks (Inst), Medicenna (Inst), Bayer (Inst), Takeda (Inst), Gilead Sciences (Inst), LegoChem Biosciences (Inst), Daiichi Sankyo (Inst)
Uncompensated Relationships: Seagen, Zymeworks, Lilly, Roche/Genentech, Repare Therapeutics, Janssen Oncology
Andrés Cervantes
Consulting or Advisory Role: Merck Serono (Inst), Amgen (Inst), Roche (Inst), Transgene (Inst), Foundation Medicine (Inst)
Research Funding: Novartis (Inst), BeiGene (Inst), FibroGen (Inst), Astellas Pharma (Inst), MedImmune (Inst), Amgen (Inst), Actuate Therapeutics (Inst), Adaptimmune (Inst), AstraZeneca Spain (Inst), Amcure (Inst), Bayer (Inst), BMSi (Inst), Lilly (Inst), Genentech (Inst), Merck Serono (Inst), Natera (Inst), MSD (Inst), Servier (Inst), Sierra Oncology (Inst), Takeda (Inst)
Other Relationship: Cancer Treatment Reviews, Annals of Oncology, ESMO Open
Valentina Gambardella
Consulting or Advisory Role: Boehringer Ingelheim
Research Funding: Genentech (Inst), Merck Serono (Inst), Roche (Inst), BeiGene (Inst), Bayer (Inst), Servier (Inst), Lilly (Inst), Novartis (Inst), Takeda (Inst), Astellas Pharma (Inst), FibroGen (Inst), Amcure (Inst), Natera (Inst), Sierra Oncology (Inst), AstraZeneca (Inst), MedImmune (Inst), Bristol Myers Squibb (Inst), MSD (Inst)
Travel, Accommodations, Expenses: Boehringer Ingelheim
Erika Hamilton
Consulting or Advisory Role: Pfizer (Inst), Genentech/Roche (Inst), Lilly (Inst), Daiichi Sankyo (Inst), Mersana (Inst), AstraZeneca (Inst), Novartis (Inst), Ellipses Pharma (Inst), Olema Pharmaceuticals (Inst), Stemline Therapeutics (Inst), Tubulis (Inst), Verascity Science (Inst), Theratechnologies (Inst), Gilead Sciences (Inst), Jazz Pharmaceuticals (Inst), Medical Pharma Services (Inst), Zentalis Pharmaceuticals (Inst), Greenwich LifeSciences (Inst), Jassen (Inst), Loxo (Inst), Orum Therapeutics (Inst), Relay Therapeutics (Inst), SeaGen (Inst)
Research Funding: AstraZeneca (Inst), Hutchison MediPharma (Inst), OncoMed (Inst), MedImmune (Inst), Stem CentRx (Inst), Genentech/Roche (Inst), Curis (Inst), Verastem (Inst), Zymeworks (Inst), Syndax (Inst), Lycera (Inst), Rgenix (Inst), Novartis (Inst), Mersana (Inst), Millennium (Inst), TapImmune Inc (Inst), Lilly (Inst), Pfizer (Inst), Tesaro (Inst), Boehringer Ingelheim (Inst), H3 Biomedicine (Inst), Radius Health (Inst), Acerta Pharma (Inst), Macrogenics (Inst), AbbVie (Inst), Immunomedics (Inst), Fujifilm (Inst), eFFECTOR Therapeutics (Inst), Merus (Inst), Nucana (Inst), Regeneron (Inst), Leap Therapeutics (Inst), Taiho (Inst), EMD Serono (Inst), Daiichi Sankyo (Inst), ArQule (Inst), Syros (Inst), Clovis (Inst), CytomX (Inst), InventisBio (Inst), Deciphera (Inst), Sermonix Pharmaceuticals (Inst), Sutro (Inst), Zenith Epigenetics (Inst), Arvinas (Inst), Harpoon (Inst), Black Diamond (Inst), Orinove (Inst), Molecular Templates (Inst), Seagen (Inst), Compugen (Inst), G1 Therapeutics (Inst), Karyopharm (Inst), Dana Farber Cancer Hospital (Inst), Onconova Therapeutics (Inst), Shattuck Labs (Inst), PharmaMar (Inst), Olema (Inst), Immunogen (Inst), Plexxikon (Inst), Amgen (Inst), AKESOBIO Australia (Inst), ADC Therapeutics (Inst), AtlasMedx (Inst), Aravive (Inst), Ellipses Pharma (Inst), Incyte (Inst), MabSpace Biosciences (Inst), ORIC Pharmaceuticals (Inst), Pieris Pharmaceuticals (Inst), Pionyr Immunotherapeutics (Inst), Repertoire Immune Medicines (Inst), Treadwell Therapeutics (Inst), Jacobio (Inst), Accutar Biotech (Inst), Artios (Inst), Bliss Biopharmaceutical (Inst), Cascadian Therapeutics (Inst), Dantari (Inst), Duality Biologics (Inst), Elucida Oncology (Inst), Infinity Pharmaceuticals (Inst), Relay Therapeutics (Inst), Tolmar (Inst), Torque (Inst), BeiGene (Inst), Context Therapeutics (Inst), K-Group Beta (Inst), Kind Pharmaceuticals (Inst), Loxo Oncology (Inst), Oncothyreon (Inst), Orum Therapeutics (Inst), Prelude Therapeutics (Inst), ProfoundBio (Inst), Cullinan (Inst)
Antoine Italiano
Honoraria: Bayer, Daiichi Sankyo, Lilly, Epizyme, Novartis, Roche, Ipsen
Consulting or Advisory Role: Roche, Daiichi Sankyo, Immune Design, Epizyme, Bayer, Lilly
Research Funding: BMS, Roche, Bayer, AstraZeneca/MedImmune, PharmaMar, MSD Oncology, Merck Serono
Patents, Royalties, Other Intellectual Property: BMS
Kevin Kalinsky
Employment: Grail, EQRx
Stock and Other Ownership Interests: Grail, EQRx
Consulting or Advisory Role: Lilly, Novartis, AstraZeneca, Genentech/Roche, Immunomedics, Merck, Seagen, Oncosec, 4D Pharma, Daiichi Sankyo/Astra Zeneca, Puma Biotechnology, Mersana, Menarini Silicon Biosystems, Myovant Sciences, Takeda, Prelude Therapeutics, RayzeBio, eFFECTOR Therapeutics, Cullinan Oncology
Research Funding: Novartis (Inst), Genentech/Roche (Inst), Lilly (Inst), Seagen (Inst), AstraZeneca (Inst), Daichi Sankyo (Inst), Ascentage Pharma (Inst)
Other Relationship: Immunomedics, Genentech
Ian E. Krop
Employment: PureTech
Leadership: PureTech
Stock and Other Ownership Interests: PureTech
Honoraria: AstraZeneca, Daiichi Sankyo
Consulting or Advisory Role: Genentech/Roche, Seagen, Daiichi Sankyo, Merck, AstraZeneca, Novartis
Research Funding: Genentech (Inst), Pfizer (Inst), Macrogenics (Inst)
Patents, Royalties, Other Intellectual Property: Anti-murine CD19 monoclonal antibody licensed to PharMingen
Mafalda Oliveira
Honoraria: Roche, Pfizer, Eisai Europe, SeaGen, Gilead Sciences, AstraZeneca, Lilly, Medscape
Consulting or Advisory Role: Roche/Genentech, AstraZeneca, Seagen, ITeos Therapeutics, Daiichi Sankyo/Astra Zeneca, Gilead Sciences, Relay Therapeutics, Cureo Science, iOne, Lilly, MSD, Pfizer
Research Funding: Roche/Genentech (Inst), AstraZeneca (Inst), Seagen (Inst), Boehringer Ingelheim (Inst), GlaxoSmithKline (Inst), Zenith Epigenetics (Inst), Gilead Sciences (Inst), Ayala Pharmaceuticals (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Eisai, Pierre Fabre, Gilead Sciences, AstraZeneca Spain
Uncompensated Relationships: Head of the SOLTI Breast Cancer Research Group
Peter Schmid
Honoraria: AstraZeneca, Bayer, Boehringer Ingelheim, Merck, Novartis, Pfizer, Puma Biotechnology, Roche, Eisai, Celgene, Genentech, Gilead Sciences, Sanofi, Stemline Therapeutics
Consulting or Advisory Role: Genentech/Roche, AstraZeneca, Merck, Boehringer Ingelheim, Bayer, Pfizer, Novartis, Eisai, Celgene, Biontech
Research Funding: AstraZeneca (Inst), Astellas Pharma (Inst), Genentech (Inst), Novartis (Inst), Roche (Inst)
Cristina Saura
Honoraria: AstraZeneca, Daiichi Sankyo/Astra Zeneca, Eisai Europe, Ltd, Zymeworks, Puma Biotechnology, Roche Pharma AG, Seagen, Solti, Phillips Health Works
Consulting or Advisory Role: AstraZeneca, Daiichi Sankyo, Eisai, MediTech, Novartis, Pfizer, Philips Healthcare, Pierre Fabre, Puma Biotechnology, Roche, SeaGen, Gilead Sciences, Pint Pharma, Synthon, Zymeworks, Pharmalex
Speakers' Bureau: AstraZeneca, Daiichi Sankyo/Astra Zeneca, Pfizer, Pierre Fabre, Puma Biotechnology, Seagen, Exeter Pharmaceuticals, Lilly
Research Funding: Puma Biotechnology (Inst), Roche (Inst), AstraZeneca (Inst), Bayer (Inst), Boehringer Ingelheim (Inst), Bristol Myers Squibb (BMS) (Inst), CytomX Therapeutics (Inst), Daiichi Sakyo (Inst), Genentech (Inst), GlaxoSmithKline (Inst), InnoUp (Inst), Lilly (Inst), Macrogenics (Inst), Menarini (Inst), Merus (Inst), Novartis (Inst), Pfizer (Inst), Sanofi/Aventis (Inst), Seagen (Inst)
Travel, Accommodations, Expenses: Pfizer, Novartis, Roche, AstraZeneca, Puma Biotechnology, Daiichi Sankyo, Eisai Europe
Nicholas C. Turner
Consulting or Advisory Role: Novartis, AstraZeneca, Pfizer, Merck Sharp & Dohme, Lilly, Repare Therapeutics, Roche, GlaxoSmithKline, Gilead Sciences, Inivata, Guardant Health, Exact Sciences, Relay Therapeutics
Research Funding: Pfizer (Inst), Roche (Inst), AstraZeneca (Inst), Guardant Health (Inst), Inivata (Inst), InVitae (Inst), Personalis (Inst), Natera (Inst), Merck Sharpe and Dohme (Inst)
Sravanthi Cheeti
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Stephanie Hilz
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Research Funding: Genentech/Roche
Katherine E. Hutchinson
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Yanling Jin
Employment: Roche/Genentech
Stock and Other Ownership Interests: Roche/Genentech
Stephanie Royer-Joo
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Ubong Peters
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Noopur Shankar
Employment: Genentech
Stock and Other Ownership Interests: Roche/Genentech
Jennifer L. Schutzman
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Dejan Juric
Stock and Other Ownership Interests: Relay Therapeutics, PIC Therapeutics, Vibliome Therapeutics
Consulting or Advisory Role: Novartis, Eisai, Genentech, MapKure, Vibliome Therapeutics, PIC Therapeutics, Relay Therapeutics, AstraZeneca, Lilly, Pfizer
Research Funding: Novartis (Inst), Genentech (Inst), Takeda (Inst), Eisai (Inst), Amgen (Inst), Syros Pharmaceuticals (Inst), InventisBio (Inst), Infinity Pharmaceuticals (Inst), Takeda (Inst), Pfizer (Inst), Arvinas (Inst), Blueprint Medicines (Inst), AstraZeneca (Inst), Ribon Therapeutics (Inst), Scorpion Therapeutics
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2019 San Antonio Breast Cancer Symposium (SABCS), San Antonio, TX, December 10-14, 2019; American Association for Cancer Research, virtual, April 27-28, 2020; European Society for Medical Oncology, virtual, September 19-21, 2020; SABCS, virtual, December 8-11, 2020; SABCS, San Antonio, TX, December 7-10, 2021; and ASCO, Chicago, IL, June 3-7, 2022.
SUPPORT
Supported by Genentech, Inc, South San Francisco, CA. The authors acknowledge the Memorial Sloan Kettering Cancer Center support grant (P30 CA008748).
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
For eligible studies, qualified researchers may request access to individual patient-level clinical data through a data request platform. At the time of writing, this request platform is Vivli: https://vivli.org/ourmember/roche/. For up-to-date details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient re-identification.
AUTHOR CONTRIBUTIONS
Conception and design: Komal L. Jhaveri, Peter Schmid, Cristina Saura, Sravanthi Cheeti, Katherine E. Hutchinson, Yanling Jin, Stephanie Royer-Joo, Jennifer L. Schutzman, Dejan Juric
Provision of study materials or patients: Komal L. Jhaveri, Philippe L. Bedard, Andrés Cervantes, Erika Hamilton, Antoine Italiano, Ian E. Krop, Nicholas C. Turner, Katherine E. Hutchinson, Dejan Juric, Mafalda Oliveira, Cristina Saura, Melissa K. Accordino
Collection and assembly of data: All authors
Data analysis and interpretation: Komal L. Jhaveri, Melissa K. Accordino, Philippe L. Bedard, Andrés Cervantes, Erika Hamilton, Antoine Italiano, Kevin Kalinsky, Ian E. Krop, Mafalda Oliveira, Peter Schmid, Cristina Saura, Nicholas C. Turner, Andrea Varga, Sravanthi Cheeti, Stephanie Hilz, Katherine E. Hutchinson, Yanling Jin, Stephanie Royer-Joo, Ubong Peters, Noopur Shankar, Jennifer L. Schutzman, Dejan Juric
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase I/Ib Trial of Inavolisib Plus Palbociclib and Endocrine Therapy for PIK3CA-Mutated, Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced or Metastatic Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Komal L. Jhaveri
Consulting or Advisory Role: Novartis, Pfizer, AstraZeneca, Jounce Therapeutics, Synthon, Intellisphere, Bristol Myers Squibb, Genentech, AbbVie, Lilly, BluePrint Medicines, Seagen, Daiichi Sankyo, Biotheranostics, Sun Pharma Advanced Research Company, Taiho Oncology, Sanofi, Gilead Sciences, Scorpion Therapeutics
Research Funding: Novartis (Inst), Genentech (Inst), Debiopharm Group (Inst), ADC Therapeutics (Inst), Pfizer (Inst), Novita Pharmaceuticals (Inst), Clovis Oncology (Inst), Lilly (Inst), Zymeworks (Inst), Immunomedics (Inst), Puma Biotechnology (Inst), VelosBio/Merck (Inst), AstraZeneca (Inst), Context Therapeutics (Inst), Scorpion Therapeutics (Inst), Blueprint Medicines (Inst)
Travel, Accommodations, Expenses: Taiho Pharmaceutical, Jounce Therapeutics, Pfizer, AstraZeneca, Intellisphere, Lilly, Gilead Sciences, Genentech/Roche
Melissa K. Accordino
Honoraria: Incrowd
Research Funding: Novartis (Inst), Genentech (Inst), Roche (Inst)
Other Relationship: Disney
Philippe L. Bedard
Research Funding: Bristol Myers Squibb (Inst), Sanofi (Inst), AstraZeneca (Inst), Genentech/Roche (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Merck (Inst), Seagen (Inst), Lilly (Inst), Amgen (Inst), Bicara Therapeutics (Inst), Zymeworks (Inst), Medicenna (Inst), Bayer (Inst), Takeda (Inst), Gilead Sciences (Inst), LegoChem Biosciences (Inst), Daiichi Sankyo (Inst)
Uncompensated Relationships: Seagen, Zymeworks, Lilly, Roche/Genentech, Repare Therapeutics, Janssen Oncology
Andrés Cervantes
Consulting or Advisory Role: Merck Serono (Inst), Amgen (Inst), Roche (Inst), Transgene (Inst), Foundation Medicine (Inst)
Research Funding: Novartis (Inst), BeiGene (Inst), FibroGen (Inst), Astellas Pharma (Inst), MedImmune (Inst), Amgen (Inst), Actuate Therapeutics (Inst), Adaptimmune (Inst), AstraZeneca Spain (Inst), Amcure (Inst), Bayer (Inst), BMSi (Inst), Lilly (Inst), Genentech (Inst), Merck Serono (Inst), Natera (Inst), MSD (Inst), Servier (Inst), Sierra Oncology (Inst), Takeda (Inst)
Other Relationship: Cancer Treatment Reviews, Annals of Oncology, ESMO Open
Valentina Gambardella
Consulting or Advisory Role: Boehringer Ingelheim
Research Funding: Genentech (Inst), Merck Serono (Inst), Roche (Inst), BeiGene (Inst), Bayer (Inst), Servier (Inst), Lilly (Inst), Novartis (Inst), Takeda (Inst), Astellas Pharma (Inst), FibroGen (Inst), Amcure (Inst), Natera (Inst), Sierra Oncology (Inst), AstraZeneca (Inst), MedImmune (Inst), Bristol Myers Squibb (Inst), MSD (Inst)
Travel, Accommodations, Expenses: Boehringer Ingelheim
Erika Hamilton
Consulting or Advisory Role: Pfizer (Inst), Genentech/Roche (Inst), Lilly (Inst), Daiichi Sankyo (Inst), Mersana (Inst), AstraZeneca (Inst), Novartis (Inst), Ellipses Pharma (Inst), Olema Pharmaceuticals (Inst), Stemline Therapeutics (Inst), Tubulis (Inst), Verascity Science (Inst), Theratechnologies (Inst), Gilead Sciences (Inst), Jazz Pharmaceuticals (Inst), Medical Pharma Services (Inst), Zentalis Pharmaceuticals (Inst), Greenwich LifeSciences (Inst), Jassen (Inst), Loxo (Inst), Orum Therapeutics (Inst), Relay Therapeutics (Inst), SeaGen (Inst)
Research Funding: AstraZeneca (Inst), Hutchison MediPharma (Inst), OncoMed (Inst), MedImmune (Inst), Stem CentRx (Inst), Genentech/Roche (Inst), Curis (Inst), Verastem (Inst), Zymeworks (Inst), Syndax (Inst), Lycera (Inst), Rgenix (Inst), Novartis (Inst), Mersana (Inst), Millennium (Inst), TapImmune Inc (Inst), Lilly (Inst), Pfizer (Inst), Tesaro (Inst), Boehringer Ingelheim (Inst), H3 Biomedicine (Inst), Radius Health (Inst), Acerta Pharma (Inst), Macrogenics (Inst), AbbVie (Inst), Immunomedics (Inst), Fujifilm (Inst), eFFECTOR Therapeutics (Inst), Merus (Inst), Nucana (Inst), Regeneron (Inst), Leap Therapeutics (Inst), Taiho (Inst), EMD Serono (Inst), Daiichi Sankyo (Inst), ArQule (Inst), Syros (Inst), Clovis (Inst), CytomX (Inst), InventisBio (Inst), Deciphera (Inst), Sermonix Pharmaceuticals (Inst), Sutro (Inst), Zenith Epigenetics (Inst), Arvinas (Inst), Harpoon (Inst), Black Diamond (Inst), Orinove (Inst), Molecular Templates (Inst), Seagen (Inst), Compugen (Inst), G1 Therapeutics (Inst), Karyopharm (Inst), Dana Farber Cancer Hospital (Inst), Onconova Therapeutics (Inst), Shattuck Labs (Inst), PharmaMar (Inst), Olema (Inst), Immunogen (Inst), Plexxikon (Inst), Amgen (Inst), AKESOBIO Australia (Inst), ADC Therapeutics (Inst), AtlasMedx (Inst), Aravive (Inst), Ellipses Pharma (Inst), Incyte (Inst), MabSpace Biosciences (Inst), ORIC Pharmaceuticals (Inst), Pieris Pharmaceuticals (Inst), Pionyr Immunotherapeutics (Inst), Repertoire Immune Medicines (Inst), Treadwell Therapeutics (Inst), Jacobio (Inst), Accutar Biotech (Inst), Artios (Inst), Bliss Biopharmaceutical (Inst), Cascadian Therapeutics (Inst), Dantari (Inst), Duality Biologics (Inst), Elucida Oncology (Inst), Infinity Pharmaceuticals (Inst), Relay Therapeutics (Inst), Tolmar (Inst), Torque (Inst), BeiGene (Inst), Context Therapeutics (Inst), K-Group Beta (Inst), Kind Pharmaceuticals (Inst), Loxo Oncology (Inst), Oncothyreon (Inst), Orum Therapeutics (Inst), Prelude Therapeutics (Inst), ProfoundBio (Inst), Cullinan (Inst)
Antoine Italiano
Honoraria: Bayer, Daiichi Sankyo, Lilly, Epizyme, Novartis, Roche, Ipsen
Consulting or Advisory Role: Roche, Daiichi Sankyo, Immune Design, Epizyme, Bayer, Lilly
Research Funding: BMS, Roche, Bayer, AstraZeneca/MedImmune, PharmaMar, MSD Oncology, Merck Serono
Patents, Royalties, Other Intellectual Property: BMS
Kevin Kalinsky
Employment: Grail, EQRx
Stock and Other Ownership Interests: Grail, EQRx
Consulting or Advisory Role: Lilly, Novartis, AstraZeneca, Genentech/Roche, Immunomedics, Merck, Seagen, Oncosec, 4D Pharma, Daiichi Sankyo/Astra Zeneca, Puma Biotechnology, Mersana, Menarini Silicon Biosystems, Myovant Sciences, Takeda, Prelude Therapeutics, RayzeBio, eFFECTOR Therapeutics, Cullinan Oncology
Research Funding: Novartis (Inst), Genentech/Roche (Inst), Lilly (Inst), Seagen (Inst), AstraZeneca (Inst), Daichi Sankyo (Inst), Ascentage Pharma (Inst)
Other Relationship: Immunomedics, Genentech
Ian E. Krop
Employment: PureTech
Leadership: PureTech
Stock and Other Ownership Interests: PureTech
Honoraria: AstraZeneca, Daiichi Sankyo
Consulting or Advisory Role: Genentech/Roche, Seagen, Daiichi Sankyo, Merck, AstraZeneca, Novartis
Research Funding: Genentech (Inst), Pfizer (Inst), Macrogenics (Inst)
Patents, Royalties, Other Intellectual Property: Anti-murine CD19 monoclonal antibody licensed to PharMingen
Mafalda Oliveira
Honoraria: Roche, Pfizer, Eisai Europe, SeaGen, Gilead Sciences, AstraZeneca, Lilly, Medscape
Consulting or Advisory Role: Roche/Genentech, AstraZeneca, Seagen, ITeos Therapeutics, Daiichi Sankyo/Astra Zeneca, Gilead Sciences, Relay Therapeutics, Cureo Science, iOne, Lilly, MSD, Pfizer
Research Funding: Roche/Genentech (Inst), AstraZeneca (Inst), Seagen (Inst), Boehringer Ingelheim (Inst), GlaxoSmithKline (Inst), Zenith Epigenetics (Inst), Gilead Sciences (Inst), Ayala Pharmaceuticals (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Eisai, Pierre Fabre, Gilead Sciences, AstraZeneca Spain
Uncompensated Relationships: Head of the SOLTI Breast Cancer Research Group
Peter Schmid
Honoraria: AstraZeneca, Bayer, Boehringer Ingelheim, Merck, Novartis, Pfizer, Puma Biotechnology, Roche, Eisai, Celgene, Genentech, Gilead Sciences, Sanofi, Stemline Therapeutics
Consulting or Advisory Role: Genentech/Roche, AstraZeneca, Merck, Boehringer Ingelheim, Bayer, Pfizer, Novartis, Eisai, Celgene, Biontech
Research Funding: AstraZeneca (Inst), Astellas Pharma (Inst), Genentech (Inst), Novartis (Inst), Roche (Inst)
Cristina Saura
Honoraria: AstraZeneca, Daiichi Sankyo/Astra Zeneca, Eisai Europe, Ltd, Zymeworks, Puma Biotechnology, Roche Pharma AG, Seagen, Solti, Phillips Health Works
Consulting or Advisory Role: AstraZeneca, Daiichi Sankyo, Eisai, MediTech, Novartis, Pfizer, Philips Healthcare, Pierre Fabre, Puma Biotechnology, Roche, SeaGen, Gilead Sciences, Pint Pharma, Synthon, Zymeworks, Pharmalex
Speakers' Bureau: AstraZeneca, Daiichi Sankyo/Astra Zeneca, Pfizer, Pierre Fabre, Puma Biotechnology, Seagen, Exeter Pharmaceuticals, Lilly
Research Funding: Puma Biotechnology (Inst), Roche (Inst), AstraZeneca (Inst), Bayer (Inst), Boehringer Ingelheim (Inst), Bristol Myers Squibb (BMS) (Inst), CytomX Therapeutics (Inst), Daiichi Sakyo (Inst), Genentech (Inst), GlaxoSmithKline (Inst), InnoUp (Inst), Lilly (Inst), Macrogenics (Inst), Menarini (Inst), Merus (Inst), Novartis (Inst), Pfizer (Inst), Sanofi/Aventis (Inst), Seagen (Inst)
Travel, Accommodations, Expenses: Pfizer, Novartis, Roche, AstraZeneca, Puma Biotechnology, Daiichi Sankyo, Eisai Europe
Nicholas C. Turner
Consulting or Advisory Role: Novartis, AstraZeneca, Pfizer, Merck Sharp & Dohme, Lilly, Repare Therapeutics, Roche, GlaxoSmithKline, Gilead Sciences, Inivata, Guardant Health, Exact Sciences, Relay Therapeutics
Research Funding: Pfizer (Inst), Roche (Inst), AstraZeneca (Inst), Guardant Health (Inst), Inivata (Inst), InVitae (Inst), Personalis (Inst), Natera (Inst), Merck Sharpe and Dohme (Inst)
Sravanthi Cheeti
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Stephanie Hilz
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Research Funding: Genentech/Roche
Katherine E. Hutchinson
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Yanling Jin
Employment: Roche/Genentech
Stock and Other Ownership Interests: Roche/Genentech
Stephanie Royer-Joo
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Ubong Peters
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Noopur Shankar
Employment: Genentech
Stock and Other Ownership Interests: Roche/Genentech
Jennifer L. Schutzman
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Dejan Juric
Stock and Other Ownership Interests: Relay Therapeutics, PIC Therapeutics, Vibliome Therapeutics
Consulting or Advisory Role: Novartis, Eisai, Genentech, MapKure, Vibliome Therapeutics, PIC Therapeutics, Relay Therapeutics, AstraZeneca, Lilly, Pfizer
Research Funding: Novartis (Inst), Genentech (Inst), Takeda (Inst), Eisai (Inst), Amgen (Inst), Syros Pharmaceuticals (Inst), InventisBio (Inst), Infinity Pharmaceuticals (Inst), Takeda (Inst), Pfizer (Inst), Arvinas (Inst), Blueprint Medicines (Inst), AstraZeneca (Inst), Ribon Therapeutics (Inst), Scorpion Therapeutics
No other potential conflicts of interest were reported.
REFERENCES
- 1. DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 2. Burstein HJ, Somerfield MR, Barton DL, et al. Endocrine treatment and targeted therapy for hormone receptor–positive, human epidermal growth factor receptor 2–negative metastatic breast cancer: ASCO guideline update. J Clin Oncol. 2021;39:3959–3977. doi: 10.1200/JCO.21.01392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31:1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gennari A, André F, Barrios CH, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32:1475–1495. doi: 10.1016/j.annonc.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 5.https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/218197s000lbl.pdf AstraZeneca Pharmaceuticals LP: TRUQAP™ (capivasertib). Prescribing information, 2023.
- 6. Hanker AB, Sudhan DR, Arteaga CL. Overcoming endocrine resistance in breast cancer. Cancer Cell. 2020;37:496–513. doi: 10.1016/j.ccell.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anderson EJ, Mollon LE, Dean JL, et al. A systematic review of the prevalence and diagnostic workup of PIK3CA mutations in HR+/HER2- metastatic breast cancer. Int J Breast Cancer. 2020;2020:3759179. doi: 10.1155/2020/3759179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller TW, Balko JM, Arteaga CL. Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol. 2011;29:4452–4461. doi: 10.1200/JCO.2010.34.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. LoRusso PM. Inhibition of the PI3K/AKT/mTOR pathway in solid tumors. J Clin Oncol. 2016;34:3803–3815. doi: 10.1200/JCO.2014.59.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martínez-Sáez O, Chic N, Pascual T, et al. Frequency and spectrum of PIK3CA somatic mutations in breast cancer. Breast Cancer Res. 2020;22:45. doi: 10.1186/s13058-020-01284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–643. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 12. Fillbrunn M, Signorovitch J, André F, et al. PIK3CA mutation status, progression and survival in advanced HR+/HER2- breast cancer: A meta-analysis of published clinical trials. BMC Cancer. 2022;22:1002. doi: 10.1186/s12885-022-10078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Leary B, Cutts RJ, Liu Y, et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov. 2018;8:1390–1403. doi: 10.1158/2159-8290.CD-18-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnston SR. Enhancing the efficacy of hormonal agents with selected targeted agents. Clin Breast Cancer. 2009;9:S28–S36. doi: 10.3816/CBC.2009.s.003. suppl 1. [DOI] [PubMed] [Google Scholar]
- 15. O'Brien NA, McDermott MSJ, Conklin D, et al. Targeting activated PI3K/mTOR signaling overcomes acquired resistance to CDK4/6-based therapies in preclinical models of hormone receptor-positive breast cancer. Breast Cancer Res. 2020;22:89. doi: 10.1186/s13058-020-01320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herrera-Abreu MT, Palafox M, Asghar U, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 2016;76:2301–2313. doi: 10.1158/0008-5472.CAN-15-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vora SR, Juric D, Kim N, et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell. 2014;26:136–149. doi: 10.1016/j.ccr.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Juric D, Ismail-Khan R, Campone M, et al. Phase Ib/II study of ribociclib and alpelisib and letrozole in ER+, HER2– breast cancer: Safety, preliminary efficacy and molecular analysis. Cancer Res. 2016;76 abstr P3-14-01. [Google Scholar]
- 19. Wesolowski R, Rugo H, Stringer-Reasor E, et al. Updated results of a phase 1b study of gedatolisib plus palbociclib and endocrine therapy in women with hormone receptor positive advanced breast cancer: Subgroup analysis by PIK3CA mutation status. Cancer Res. 2023;83 abstr PD13-05. [Google Scholar]
- 20. André F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-mutated, hormone receptor–positive advanced breast cancer. N Engl J Med. 2019;380:1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 21. Cheung YM, Cromwell GE, Tolaney SM, et al. Factors leading to alpelisib discontinuation in patients with hormone receptor positive, human epidermal growth factor receptor-2 negative breast cancer. Breast Cancer Res Treat. 2022;192:303–311. doi: 10.1007/s10549-021-06476-1. [DOI] [PubMed] [Google Scholar]
- 22. Bello Roufai D, Gonçalves A, De La Motte Rouge T, et al. Alpelisib and fulvestrant in PIK3CA-mutated hormone receptor-positive HER2-negative advanced breast cancer included in the French early access program. Oncogene. 2023;42:1951–1956. doi: 10.1038/s41388-022-02585-3. [DOI] [PubMed] [Google Scholar]
- 23. Hong R, Edgar K, Song K, et al. GDC-0077 is a selective PI3Kalpha inhibitor that demonstrates robust efficacy in PIK3CA mutant breast cancer models as a single agent and in combination with standard of care therapies. Cancer Res. 2018;78 (abstr PD4-14) [Google Scholar]
- 24. Edgar K, Hong R, Song K, et al. GDC-0077 is a selective PI3K alpha inhibitor with robust efficacy in PIK3CA mutant hormone-positive breast cancer models. Cancer Res. 2020;80 abstr P3-11-23. [Google Scholar]
- 25. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Center for Drug Evaluation and Research (CDER) https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207103orig1s000clinpharmr.pdf Clinical pharmacology and biopharmaceutics review(s). Application number: 207103Orig1s000, 2014.
- 27. Awada A, Cardoso F, Fontaine C, et al. The oral mTOR inhibitor RAD001 (everolimus) in combination with letrozole in patients with advanced breast cancer: Results of a phase I study with pharmacokinetics. Eur J Cancer. 2008;44:84–91. doi: 10.1016/j.ejca.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 28.AstraZeneca AB https://www.ema.europa.eu/en/documents/product-information/faslodex-epar-product-information_en.pdf Faslodex (fulvestrant). Summary of product characteristics, 2022.
- 29.AstraZeneca UK Limited https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/021344s044lbl.pdf FASLODEX®(fulvestrant). Prescribing information, 2021.
- 30. Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lopez JS, SelviMiralles M, Ameratunga M, et al. PIPA: A phase Ib study of selective ß-isoform sparing phosphatidylinositol 3-kinase (PI3K) inhibitor taselisib (T) plus palbociclib (P) in patients (pts) with advanced solid cancers—Safety, tolerability, pharmacokinetic (PK), and pharmacodynamic (PD) analysis of the doublet combination. J Clin Oncol. 2019;37 suppl 15; abstr 3087. [Google Scholar]
- 32. Hurvitz SA, Andre F, Cristofanilli M, et al. A phase 3 study of gedatolisib plus fulvestrant with and without palbociclib in patients with HR+/HER2- advanced breast cancer previously treated with a CDK4/6 inhibitor plus a nonsteroidal aromatase inhibitor (VIKTORIA-1) J Clin Oncol. 2023;41 suppl 16; abstr TPS1118. [Google Scholar]
- 33. Hamilton E, Schiavon G, Grinsted LM, et al. CAPItello-292: A phase 1b/3 study of capivasertib, palbociclib and fulvestrant versus placebo, palbociclib and fulvestrant in HR+/HER2− advanced breast cancer. Ann Oncol. 2021;32 (abstr 338TiP) [Google Scholar]
- 34. Layman R, Wesolowski R, Han H, et al. Phase Ib expansion study of gedatolisib in combination with palbociclib and endocrine therapy in women with ER+ metastatic breast cancer. Cancer Res. 2022;82 abstr PD13-02. [Google Scholar]
- 35. Turner NC, Oliveira M, Howell SJ, et al. Capivasertib in hormone receptor–positive advanced breast cancer. N Engl J Med. 2023;388:2058–2070. doi: 10.1056/NEJMoa2214131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Juric D, Kalinsky K, Oliveira M, et al. A first-in-human phase Ia dose escalation study of GDC-0077, a p110a-selective and mutant-degrading PI3K inhibitor, in patients with PIK3CA-mutant solid tumors. Cancer Res. 2020;80 abstr OT1-08-04. [Google Scholar]
- 37.Jhaveri KL, Im S-A, Saura C, et al. Inavolisib or placebo in combination with palbociclib and fulvestrant in patients with PIK3CA-mutated, hormone receptor-positive, HER2-negative locally advanced or metastatic breast cancer: Phase III INAVO120 primary analysis. Presented at the Oral presentation at San Antonio Breast Cancer Symposium (SABCS), San Antonio, TX, December 5-9, 2023 (abstr GS03-13)
- 38. Clark TA, Chung JH, Kennedy M, et al. Analytical validation of a hybrid capture-based next-generation sequencing clinical assay for genomic profiling of cell-free circulating tumor DNA. J Mol Diagn. 2018;20:686–702. doi: 10.1016/j.jmoldx.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
For eligible studies, qualified researchers may request access to individual patient-level clinical data through a data request platform. At the time of writing, this request platform is Vivli: https://vivli.org/ourmember/roche/. For up-to-date details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient re-identification.