Abstract
Background:
Macrophages play an important role in the symptoms and structural progression of periodontitis, and are receiving increasing attention. In recent years, research has shown significant progress in macrophage associated periodontitis. However, there is still lack of comprehensive and methodical bibliometric analysis in this domain. Therefore, this research aims to describe the state of the research and current research hotspots of macrophage associated periodontitis from the perspective of bibliometrics.
Methods:
This study collected and screened a total of 1424 articles on macrophage associated periodontitis retrieved between 2004 and 2023 from Web of Science Core Collection database. Use Citespace (6.1. R6), Bibliometrix-R (4.1.3), VOSviewer (1.6.19), and Graphpad Prism8 software to analyze and plot countries/regions, institutions, journals, authors, literature, and keywords to explore the research hotspots and development trends of macrophage associated periodontitis.
Result:
After analysis, the amount of macrophage associated periodontitis publications has been rising consistently over time, with China having the most publications (29.32%). 3 countries accounted for 65.57% of the total publications: the United States, China, and Japan, occupying a dominant position in this research field. China publications have the fastest growth rate and played a driving role. The most productive institution is the Sichuan University in China. Journal of Periodontal Research is highly popular in the field of macrophage associated periodontitis, with the highest number of publications. Grenier, Daniel is the most prolific author. Inflammation and Bone Loss in Periodontal Disease are the most cited literature. “Biological pathogenic factors,” “immune regulation,” “mechanism research,” “susceptibility factor research,” “pathological processes and molecular correlation,” “pathological characteristics,” “inflammatory response” are the main keyword groups in this field.
Conclusion:
This study systematically analyzes and describes the development process, direction, and hotspots of macrophage associated periodontitis using bibliometric methods, providing a reference for future researchers who continue to study macrophage associated periodontitis.
Keywords: bibliometric, Citespace, macrophages, periodontitis, VOSviewer
1. Introduction
Periodontitis remains one of the most common oral diseases. Periodontitis, holds the status of being the sixth most common illness in humans globally, affecting an approximate 11.2% of the world adult populace.[1] From 1990 to 2019, severe periodontitis’ incidence, prevalence, and Disability Adjusted Life Year rates showed rising trends throughout Asia, with notable regional disparities across the various nations.[2] This will lead to an increase in economic burden and a decrease in quality of life, causing long-term pain for patients.[3] Periodontitis is commonly characterized by gingival inflammation, clinical attachment loss, alveolar bone loss seen on imaging, deep probing depth, movement, bleeding while probing, and pathological migration.[4,5] In recent years, although measures such as home care, scaling, and root planning have been able to alleviate symptoms and delay disease progression. However, the delicate balance between periodontal microbiota and immunity[6] in patients with periodontitis makes it difficult to ensure the prognosis and functional recovery of patients with periodontitis.
The process that causes periodontitis is attributed to a local ecological dysbiosis microbial community. This microbial community stimulates host immune responses that are impacted by genetic, environmental, and local microbial community pathogenicity.[7] Therefore, we still need research on the pathological and physiological basis of periodontitis and good prognostic methods.
In recent years, the role of macrophages in periodontitis has received increasing attention. Innate immune cells called macrophages, or Macs for short are located on the surface of the epithelium, which respond quickly to infections.[8] Macrophages are highly efficient antigen-presenting cells, especially skilled at triggering T cells, amplifying the immune system by phagocytosing pathogens, phagocytosing,[9–11] and secreting cytokines, inducing and expanding inflammation, triggering regional inflammatory reactions and causing tissue deterioration. Macrophages have the ability to create particular cytokines and chemotactic chemicals, identify pathogen associated molecular patterns through TLRs, and attract nonresident neutrophils and other white blood cells to take part in defense responses.[12] Additionally, macrophages, a subtype of plastic cell, are classified into M1 and M2 types.[13,14] Classically activated macrophages, another name for M1 macrophages, can be stimulated and activated by LPS (lipopolysaccharide), interferon g, reactive oxygen species, granulocyte macrophage colony stimulating factor, microRNAs, and other stimuli.[15] In periodontal tissue, the differentiation of macrophages into M1 type is mainly induced by microbial related factors dominated by interferon g and lps2 produced by Th1.[16] The activation of M1 is involved in the development of defense and inflammation, as well as in controlling immune cell processes and function.[17] Bone resorption and the M1 phenotype are tightly associated as well.[18] Selectively activated macrophages, commonly referred to as M2 macrophages, are primarily involved in the processes of tissue regeneration, inflammation resolution, and repair. They have a minor role in immune response. They are believed to have anti-inflammatory effects.[19] Macrophages can undergo alternate activation into M2 when Th2 related cytokines like IL-4 and IL-13 stimulate them.[20] M1-associated factors have the ability to modulate M2 polarization, which in turn affects Th17 and Treg cell functions related to anti-inflammatory and repair.[21] At present, the stage of action of macrophages in anti-inflammatory therapy is exactly identified as the M2 phenotype, which alternates activation phenotype.[22] In addition to encouraging bone integration and angiogenesis, the local milieu that m2 creates also contributes to the inhibition of bone production and resorption.[23] The environment of periodontal tissue was not studied for this portion of the study, but the relevant mechanisms in other types of tissue inflammation are entirely applicable to the delayed process of periodontitis, which still needs to be elucidated.
In recent years, bibliometrics has been widely used to analyze a large amount of research data. Its use of information visualization techniques and technologies allows it to show research development patterns, research hotspots, the current state of the field, and the development process in an intuitive manner. In previous studies, scholars have published bibliometric studies related to periodontitis,[24] but there is currently a lack of research reports on macrophage associated periodontitis. For the purpose of this study, we examined the development trends and research hotspots of macrophage-associated periodontitis by analyzing and visualizing pertinent literature from 2004 to 2023 using bibliometric tools like Citespace, VOSviewer, and the R software package “bibliometrix.”
2. Material and methods
2.1. Data sources and search methods
The Web of Science Core Database (WoSCC) is the world preeminent citation database. It contains conference proceedings, publications and paper records from the world most prestigious journals, including open access journals. It contains 10 distinctive indexes, including Science Citation Index Expanded, Social Sciences Citation Index, Arts & Humanities Citation Index. Conference Proceedings Citation Index-Science, Conference Proceedings Citation Index-Social Science & Humanities, Book Citation Index-Science, Book Citation Index-Social Sciences & Humanities, Emerging Sources Citation Index, Current Chemical Reactions, and Index Chemicus. Among them, Social Sciences Citation Index, Arts & Humanities Citation Index, Conference Proceedings Citation Index-Science, Conference Proceedings Citation Index-Social Science & Humanities, Book Citation Index-Science, Book Citation Index-Social Sciences & Humanities, Current Chemical Reactions, and Index Chemicus were not applicable to this study. Therefore, we chose it as the data source for bibliometric analysis. In this study, the search terms set were as follows: (TS = [periodontitis]) AND TS = (macrophage), Publication Year = 2004 to 2023, Document Types = Article OR Review Article, Language = English. After filtering, we extracted information such as (Full Record and Cited References) Author (s), Title, Source, Abstract, Keywords, etc and exported them in the form of Plain text files, which were saved in the database. Next, we will use Excel 2021 for data organization and filtering, and ultimately use Graphpad Prism8 for analysis.
2.2. Bibliometric analysis and visualization
Citespace is an information visualization program built on the Java programming language and citation analysis theory. It was created by Dr Chen Chaomei, a computer and intelligence professor at Drexel University in the United States. In this study, it was applied to collinear analysis, construction of journal double graph overlay, and timeline view. The size of a node indicates how frequently it occurs; the larger the node, the more frequently it occurs. Relationships that are cooperative, cooccurring, or co referencing are represented by the connections between nodes. Nodes of different colors represent different years; Circles of different colors from inside out represent the period from 2004 to 2023. VOSviewer, developed by van Eck and Waltman at the Technology Research Center of Leiden University in the Netherlands, is a software application utilized for the construction and visualization of bibliometric networks. In this study, it was applied to citation coupling, co-citation, or co-author relationship construction. Bibliometrix is a scientific measurement software based on R language developed by Dr Massimo Aria and others from Federick II University in Naples, Italy. This study is applied to visualize publication production between countries, draw international cooperation maps between countries, and visualize 3 field analysis. The Scholar H-Index, Journal IF (Impact Factor), and Journal Citation Report are all derived from Web of Science and included in the analysis as scientific indicators.
3. Results
3.1. Overall situation of literature
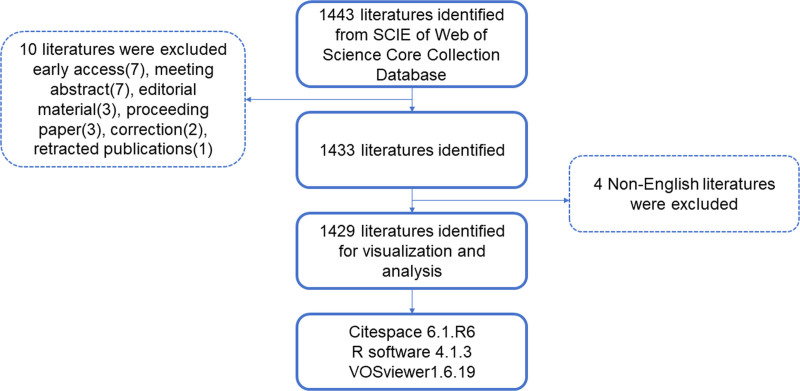
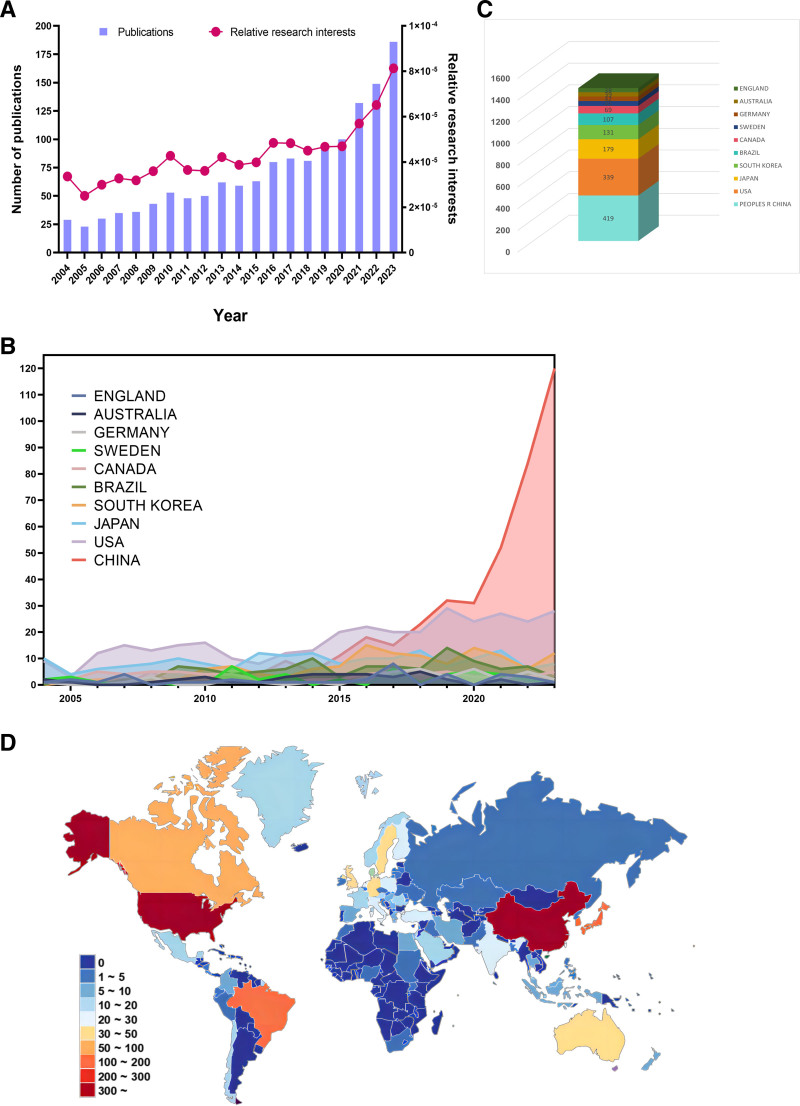
Based on the search results of WoSCC index terms, we selected and collected a total of 1443 articles. Next, we excluded 7 articles with early access, meeting abstract (7), editorial material (3), proceeding paper (3), correction (2), retracted publications (1), and 4 non-English articles. In the end, a total of 1429 articles were selected (Fig. 1). As depicted in Figure 2A, there is a gradual and steady increase in the quantity of world literature. The annual number of articles increased from 29 in 2004 to 186 in 2023, with the year with the lowest number of publications being 2005 (23 articles, 1.61%) and the year with the highest number of published studies being 2023 (186 articles, 13%) (Fig. 2A). At the same time, Research interests in this field, as indicated by relative research interest, which quantifies the annual publication count of papers in a certain field across all literature, are also experiencing an upward trend.
Figure 1.
Overview of article filtering.
Figure 2.
(A) Global number of publications related to macrophages associated periodontitis (purple bar) and related research interests (red curve), (B) annual publication volume statistics of the top 10 countries and regions in publication rankings, (C) top 10 countries and regions in publications related to macrophages associated periodontitis, (D) distribution of publications related to macrophages associated periodontitis on world maps.
The analysis results show that a total of 69 countries or regions have published in this field, with China having the largest contribution (419 articles, 29.32%), followed by the United States (339 articles, 23.72%), Japan (179 articles, 12.53%), South Korea (131 articles, 9.17%), and Brazil (107 articles, 7.49%) (Fig. 2C and D). Moreover, from Figure 2B, we can see that among the top 10 countries or regions in terms of publication volume, before 2020, the United States, Japan, and South Korea had relatively stable and overall upward trends in publication volume, while China had the fastest growth rate of publications after 2020, while other countries had relatively stable annual publication volumes. The above results indicate that research on macrophages and periodontitis has received increasing attention from scholars, and research in this field has entered a relatively rapid development stage.
3.2. Analysis of national and institutional situations
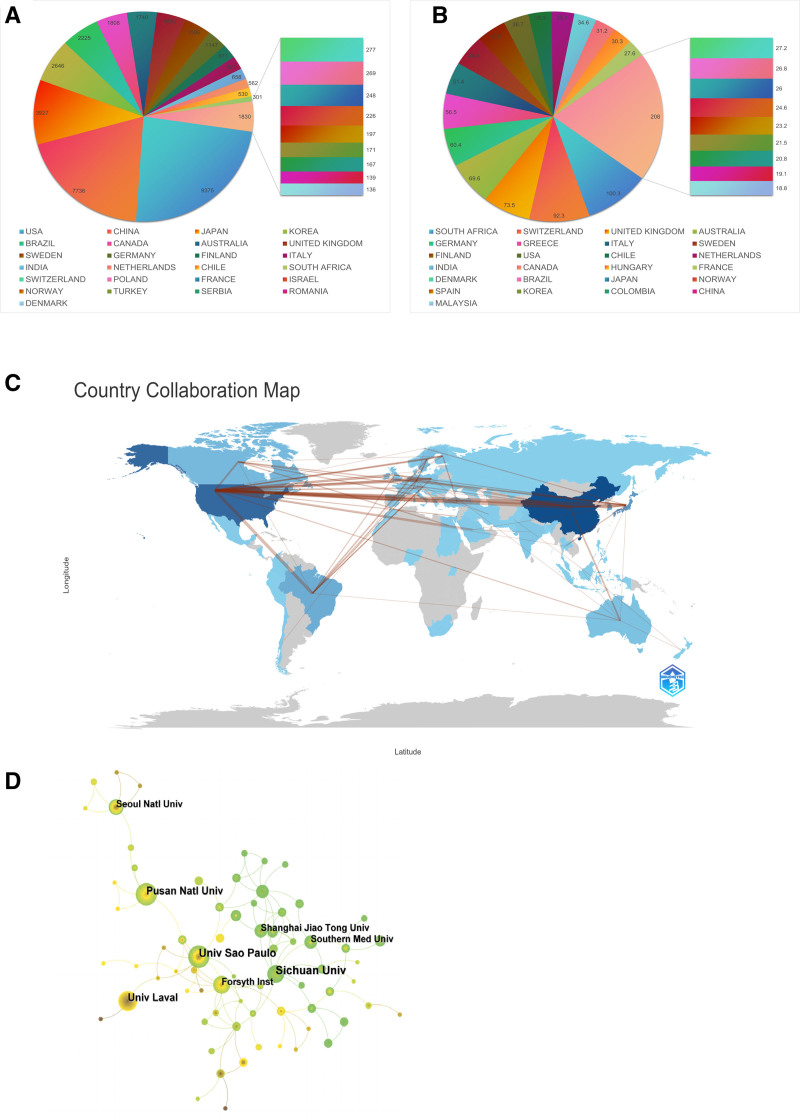
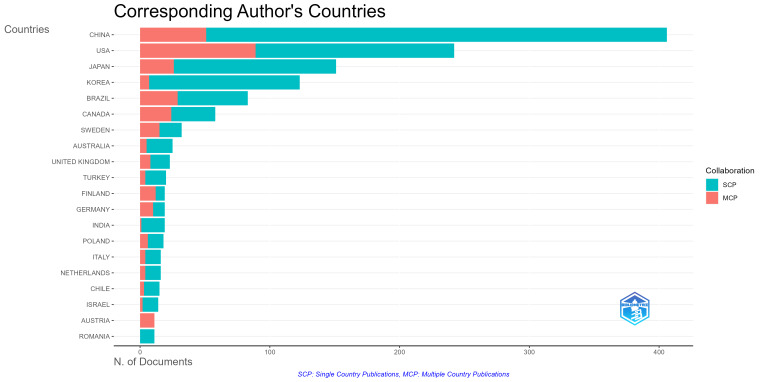
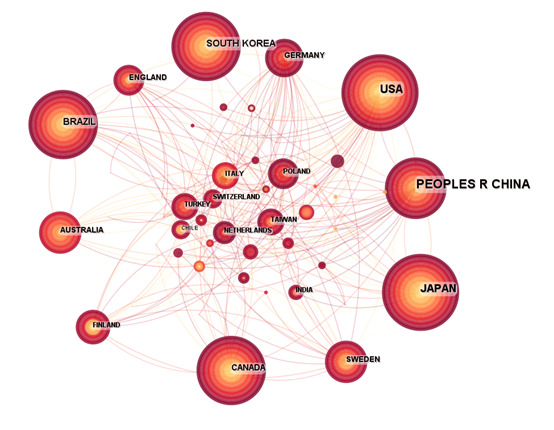
We have selected the top 25 countries and regions with the highest citation frequency and average citation frequency among the countries and regions that have made contributions in this field. From Figure 3A, it can be seen that publications in the United States have the highest citation frequency (9375 times), followed by China (7736 times), and then Japan (3927 times), South Korea (2646 times), and Brazil (2225 times). From Figure 3B, it can be seen that South Africa has the highest average citation frequency (100.3 times), followed by Switzerland (92.3 times), followed by the United Kingdom (73.5 times), Australia (69.6 times), and Germany (60.4 times). Next, we additionally examined the regions and countries in which the corresponding authors reside. The results showed that China (355 articles) had the highest number of corresponding authors in a single country publication, followed by the United States (153 authors) and Japan (125 authors). In a multi-country publication, The higher quantity of corresponding authors was found in the United States (89 authors), followed by China (51 authors) and Japan (29 authors) (Fig. S1, Supplemental Digital Content, http://links.lww.com/MD/N969). In addition, from the analysis chart of national or regional cooperation (Fig. S2, Supplemental Digital Content, http://links.lww.com/MD/N970) we can see that the United States has the highest article production and maintains strong partnerships with nations, such as Brazil, Japan, Germany, the United Kingdom, and Canada. By analyzing the world map of cooperation between countries (Fig. 3C), we can find that cooperation mainly exists in North America, Europe, and eastern Asia, with a few in South America and Oceania.
Figure 3.
(A) The top 25 countries and regions in terms of total citation of publications related to macrophages associated periodontitis, (B) the top 25 countries and regions in terms of average citation of publications related to macrophages associated periodontitis, (C) the geographical network map of macrophages associated periodontitis, (D) collaboration Institutions Analysis of Macrophages associated Periodontitis.
Table 1 lists the top 10 institutions in terms of production, with the highest production being the Sichuan University in China (53 papers), followed closely by University of São Paulo in Brazil (42 papers), and Pusan University in South Korea (40 papers) in third place. Figure 3D shows the collaborative relationships between institutions. We can see that institutions such as Sichuan University and Shanghai Jiao Tong University in China, as well as institutions such as University of Louisville and Forsyth Institute in the United States, have strong collaborative relationships.
Table 1.
Top 10 institutions in the literature on macrophages associated periodontitis published from 2004 to 2023.
| Ranking | Name | Country | Count | Percentage (%) | Centrality | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Sichuan University | China | 53 | 3.37% | 0.21 | |||||
| 2 | Universidade de São Paulo | Brazil | 42 | 3.21% | 0.12 | |||||
| 3 | Pusan National University | Korea | 40 | 3.04% | 0.10 | |||||
| 4 | Laval University | Canada | 36 | 2.57% | 0.04 | |||||
| 5 | Forsyth Institute | USA | 28 | 2.09% | 0.23 | |||||
| 6 | Seoul National University | Korea | 27 | 2.01% | 0.06 | |||||
| 7 | University of Louisville | USA | 25 | 1.85% | 0.27 | |||||
| 8 | Wuhan University | China | 25 | 1.36% | 0.01 | |||||
| 9 | Southern Medical University | China | 22 | 1.28% | 0.03 | |||||
| 10 | Shanghai Jiao Tong University | China | 21 | 1.20% | 0.03 | |||||
| Ranking | Name | Country | Count | Percentage (%) | Centrality | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Universidade de São Paulo | Brazil | 42 | 3.37% | 0.14 | |||||
| 2 | Sichuan University | China | 40 | 3.21% | 0.14 | |||||
| 3 | Pusan National University | Korea | 38 | 3.04% | 0.1 | |||||
| 4 | Laval University | Canada | 32 | 2.57% | 0.04 | |||||
| 5 | Forsyth Institute | USA | 26 | 2.09% | 0.22 | |||||
| 6 | University of Louisville | USA | 25 | 2.01% | 0.29 | |||||
| 7 | Seoul National University | Korea | 23 | 1.85% | 0.06 | |||||
| 8 | Wuhan University | China | 17 | 1.36% | 0.01 | |||||
| 9 | University of Pennsylvania | USA | 16 | 1.28% | 0.16 | |||||
| 10 | Nihon University | Japan | 15 | 1.20% | 0.05 | |||||
3.3. Analysis of journal and author situation
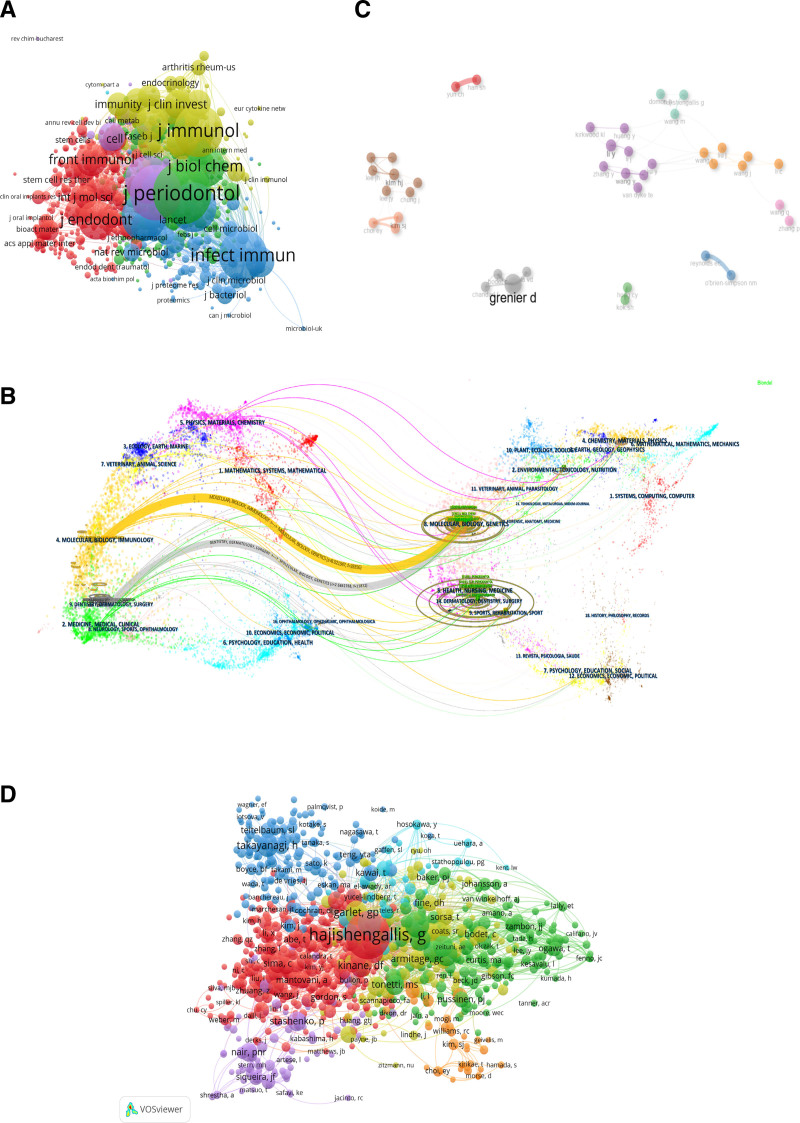
Table 2 lists the top 10 journals in terms of production volume and citation frequency. Among them, Journal of Periodontal Research (98 articles) has the highest publication volume, followed by Journal of Periodontology (95 articles) and Journal of Dental Research (55 articles); Among the top 10 journals in terms of production volume, Journal of Clinical Periodontology has the highest IF of 5.9; among the top 10 journals, 80% are in Q1, 20% are in Q2. The journal with the highest number of co citations is Journal of Periodontology (3110 times), followed by Journal of Clinical Periodontology (2433 times) and Journal of Immunology (2190 times). Among the top 10 journals with the highest number of citations, Periodontology 2000 IF is 17.5. We conducted analysis and visualization of co-cited journals based on VOSviewer, and the minimum number of citations was >10. As shown in Figure 4A, a total of 854 journals were displayed in terms of total link strength, among which Journal of Periodontology (total link strength = 169,636) had the highest total link strength, followed by Journal of Immunology (total link strength = 156,687), Journal of Clinical Periodontology (total link strength = 143451), Infection and Immunity (total link strength = 136,506), Journal of Periodontal Research (total link strength = 124,539).
Table 2.
Top 10 high-yield and cited journals related to macrophages associated periodontitis published from 2004 to 2023.
| Rank | Sources | Articles | JCR (2023) | IF (2023) |
|---|---|---|---|---|
| 1 | Journal of Periodontal Research | 98 | Q1 | 3.7 |
| 2 | Journal of Periodontology | 95 | Q1 | 4.2 |
| 3 | Journal of Dental Research | 55 | Q1 | 5.7 |
| 4 | Journal of Endodontics | 55 | Q1 | 3.5 |
| 5 | Archives of Oral Biology | 45 | Q2 | 2.2 |
| 6 | Journal of Clinical Periodontology | 41 | Q1 | 5.8 |
| 7 | PLoS ONE | 38 | Q1 | 2.9 |
| 8 | Infection and Immunity | 36 | Q2 | 2.9 |
| 9 | Oral Diseases | 27 | Q1 | 2.9 |
| 10 | Frontiers in Immunology | 26 | Q1 | 5.7 |
| Rank | Cited sources | Citations | JCR (2023) | IF (2023) |
|---|---|---|---|---|
| 1 | Journal of Periodontology | 3110 | Q1 | 4.2 |
| 2 | Journal of Clinical Periodontology | 2433 | Q1 | 5.8 |
| 3 | Journal of Immunology | 2190 | Q2 | 3.6 |
| 4 | Infection and Immunity | 2104 | Q2 | 2.9 |
| 5 | Journal of Periodontal Research | 1968 | Q1 | 3.7 |
| 6 | Periodontology 2000 | 1852 | Q1 | 17.5 |
| 7 | Journal of Dental Research | 1851 | Q1 | 5.7 |
| 8 | Journal of Biological Chemistry | 1361 | Q2 | 4.0 |
| 9 | Journal of Endodontics | 1234 | Q1 | 3.5 |
| 10 | PLoS ONE | 943 | Q1 | 2.9 |
JCR = Journal Citation Report.
Figure 4.
(A) Network diagram of journals that have been co cited >10 times, (B) the dual-map overlay of journals related to macrophages associated periodontitis, (C) collaborative network map of authors on the relationship related to macrophages associated periodontitis, (D) visualization of co cited authors in publications related to macrophages associated periodontitis.
The disciplinary distribution of academic journals is depicted by overlaying the double graphs of the journals (Fig. 4B). The left side is the cited journal, and the right side is the cited journal. The cited relationship is represented by a colored path. This map identifies 2 main citation pathways for color, which means that research published in molecular/biological/genetic journals is mainly cited in molecular/biological/immunological and Dentistry/Dermatology/Surgery.
In the past 2004 to 2023, a cumulative sum of 6145 authors contributed to the development of macrophage-associated periodontitis. Scientific productivity based on Lotka law showed that 75.7% of authors only published one paper. Table 3 lists the top 10 authors in terms of productivity, with Grenier, Daniel (28 articles) having the highest publication volume. Among the top 10 authors in terms of productivity, Van Dyke, T.E. have the highest H-index of 79. Figure 4C shows the collaboration between authors, with Kim and HJ as the core members of an 8-person group, forming the largest collaborative cluster (brown). Next, we conducted an analysis and visualization of co cited authors using VOSviewer (Fig. 4D). The lowest number of citations was >10, and a total of 997 authors were displayed. The highest total link strength was found in Hajishengallis, George (total link strength = 15,004), followed by Garlet, GP (total link strength = 4532), Darvieu, RP (total link strength = 4205), Graves, DT (total link strength = 3936), Socransky, SS (total link strength = 3854).
Table 3.
Top 10 most prolific authors published from 2004 to 2023.
| Rank | Author | Count | H-index |
|---|---|---|---|
| 1 | Grenier, Daniel | 28 | 52 |
| 2 | Hajishengallis, George N. | 16 | 74 |
| 3 | Reynolds, Eric | 14 | 74 |
| 4 | Ebersole, Jeffrey L | 14 | 57 |
| 5 | Han, Seung Hyun | 13 | 35 |
| 6 | Garlet, Gustavo Pompermaier | 13 | 44 |
| 7 | O’Brien-Simpson, Neil | 13 | 48 |
| 8 | Van Dyke, T.E. | 12 | 79 |
| 9 | Yun, Cheol-Heui | 12 | 40 |
| 10 | Chung, Jin | 12 | 24 |
3.4. Citation and co-citation analysis
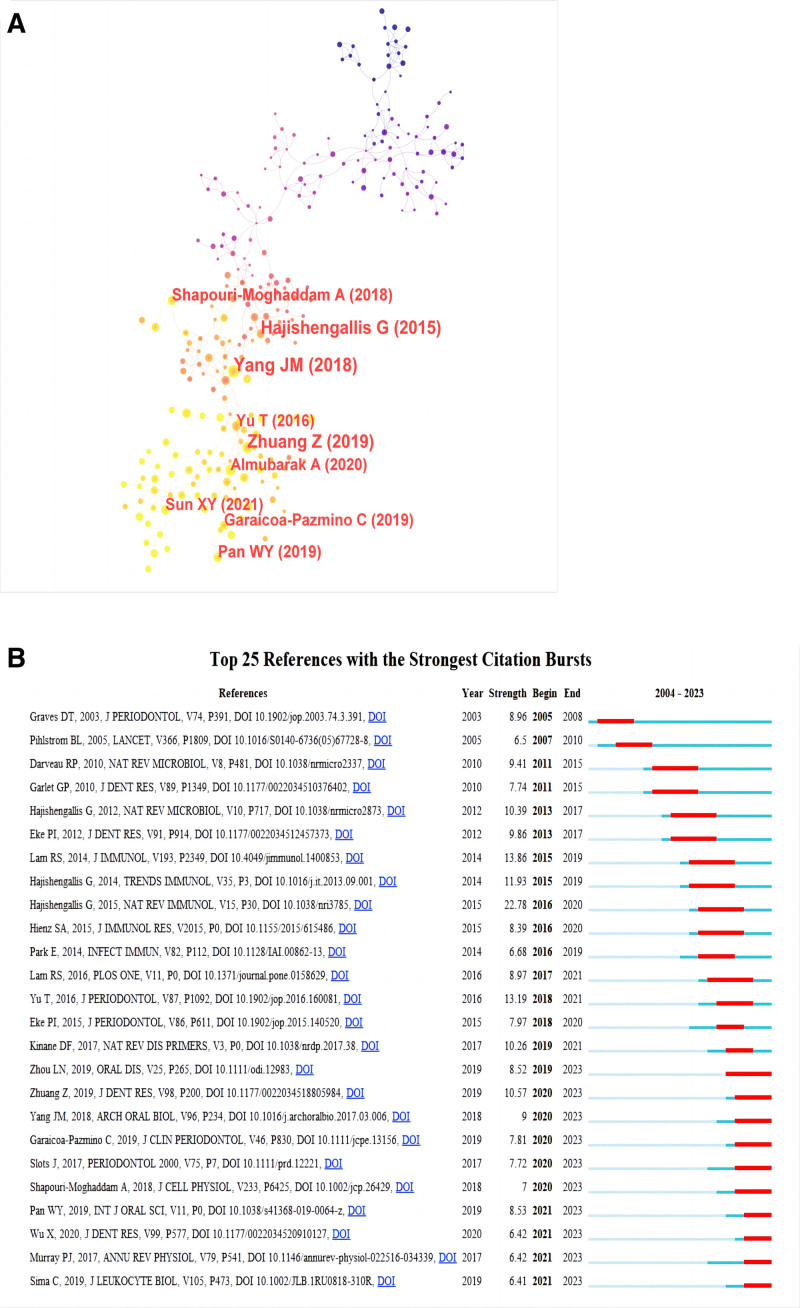
In the field of macrophage related periodontitis, a total of 498 articles have been cited more than 25 times, accounting for 34.8% of the total. We have listed the top 10 most cited literature in Table 4, with Inflammation and Bone Loss in Periodontitis Disease (479 citations) receiving the most citations, then Mechanisms of Bone Resolution in Periodontitis (459 citations) and Azithromycin: Mechanisms of action and their relevance for clinical applications (409 citations). In order to analyze the literature that has had a significant impact in the field of macrophage associated periodontitis, we conducted a co citation analysis based on Citespace. The results showed that (Fig. 5A), The article published in 2015 by Hajishengallis, George titled Periodontitis: from microbial immune subversion to systemic inflammation has significant influence. Furthermore, citation burst serves as a significant indicator that mirrors the reference literature that researchers in a specific field are interested in during a period of time. We analyzed it based on Citespace, as shown in Figure 5B. The article published in 2015 by Hajishengallis, George titled Periodontitis: from microbial immune subversion to systemic inflammation had the highest intensity (strength = 22.78).
Table 4.
The top 10 most cited literature on macrophages associated periodontitis published from 2004 to 2023.
| Rank | Title | Type | Corresponding authors | Journal | IF | Publication year | Total citations |
|---|---|---|---|---|---|---|---|
| 1 | Inflammation and Bone Loss in Periodontal Disease | Review | David L. | Journal of Periodontology | 4.2 | 2008 | 479 |
| 2 | Mechanisms of Bone Resorption in Periodontitis | Review | Ivanovski, Saso | Journal of Immunology Research | 3.5 | 2015 | 459 |
| 3 | Azithromycin: Mechanisms of action and their relevance for clinical applications | Review | Vos, Robin | Pharmacology & Therapeutics | 12 | 2014 | 409 |
| 4 | Human innate immunosenescence: causes and consequences for immunity in old age | Review | Shaw, Albert C. | Trends in Immunology | 13.1 | 2009 | 334 |
| 5 | Macrophage Polarization in Chronic Inflammatory Diseases: Killers or Builders? | Review | Mortara, Lorenzo | Journal of Immunology Research | 3.5 | 2018 | 325 |
| 6 | The clinical importance of emerging Campylobacter species | Review | Man, Si Ming | Nature Reviews Gastroenterology & Hepatology | 45.9 | 2011 | 324 |
| 7 | Cultivation of a human-associated TM7 phylotype reveals a reduced genome and epibiotic parasitic lifestyle | Article | Shi, Wenyuan | Proceedings of The National Academy of Sciences of The United States of America | 9.4 | 2015 | 310 |
| 8 | Host response mechanisms in periodontal diseases | Review | Gamonal, Jorge | Journal of Applied Oral Science | 2.2 | 2015 | 280 |
| 9 | How has research into cytokine interactions and their role in driving immune responses impacted our understanding of periodontitis? | Review | Taylor, John J. | Journal of Clinical Periodontology | 5.8 | 2011 | 261 |
| 10 | Optimized Platelet-Rich Fibrin With the Low-Speed Concept: Growth Factor Release, Biocompatibility, and Cellular Response | Article | Joseph Choukroun | Journal of Periodontology | 4.2 | 2017 | 251 |
Figure 5.
(A) Network visualization of co-cited literature on macrophages associated periodontitis related publications, (B) the top 25 most frequently cited articles related to macrophages associated periodontitis.
3.5. Keyword and hotspot analysis
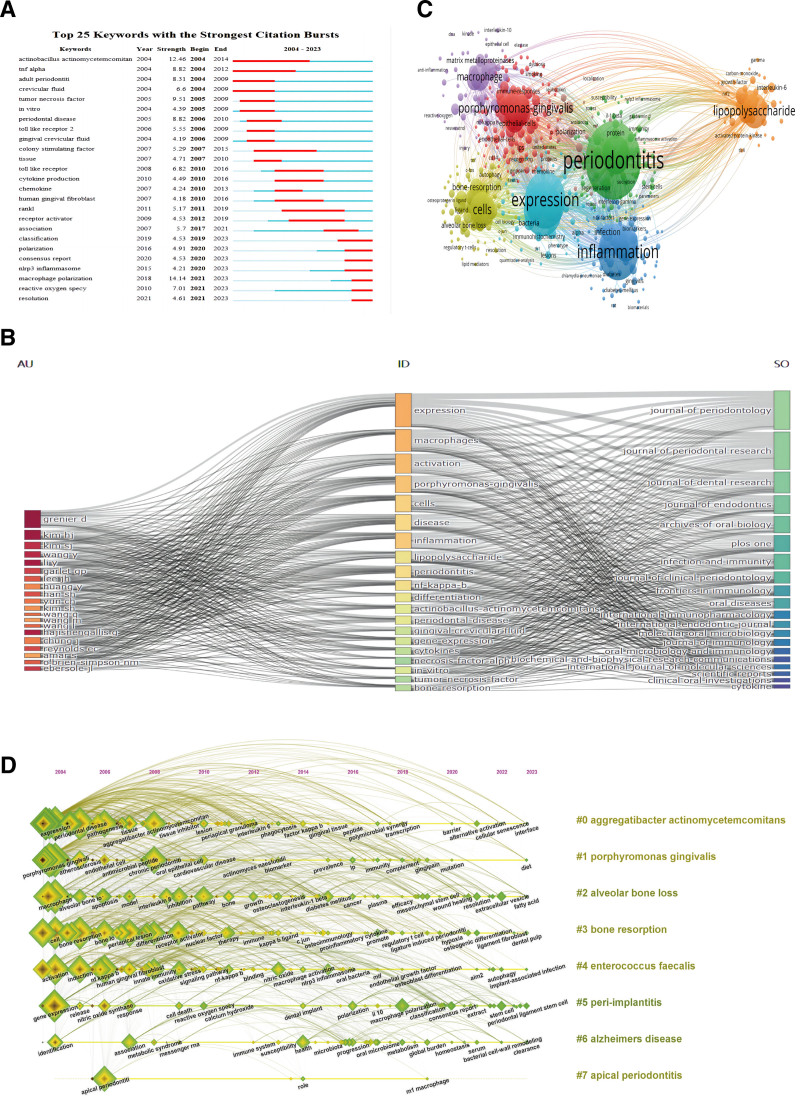
As described earlier, keyword burst refers to the extremely high frequency of appearance in a published article within a short period of time, indicating the importance and attention of keywords in the research field. As depicted in Figure 6A, we analyzed the top 25 keywords with the strongest citation burst based on Citespace. The word with the highest burst intensity was “macroscopic polarization” (strength = 14.14), and the word with the longest duration was “actinnobacillus actinomycetemcomitan,” which lasted from 2004 to 2014. More importantly, from 2020 to 2023, the citation frequency of “macrophage polarization,” “nlrp3 inflammasome,” and “reactive oxygen specy” was relatively high, which may indicate that “macrophage polarization,” “nlrp3 inflammasome,” and “reactive oxygen specy” may be future research hotspots in the field of macrophage related periodontitis. Figure 6B shows a three-field plot that represents the relationship between keywords, authors, and journals, which is represented by how strong the relationship is. “Expression” is the most commonly occurring keyword, followed by “macrophages,” “activation,” “porphyromonas gingivalis,” and so on. Authors Kim HJ and Grenier D have a relatively strong correlation with keywords such as “expression” and “metaphors”; correspondingly, it has the strongest connection with Journal of Periodontology and Journal of Periodontal Research.
Figure 6.
(A) The top 25 most cited keywords based on Citespace, (B) annotation of 3 field images for keyword plus: three-field plot for keyword analysis: (middle: keywords; left field: authors; right outer column: journals), (C) the frequency of the keyword map related to macrophages associated periodontitis is represented by point size, and the keywords in the research field are mainly divided into 7 clusters: Cluster 1 (red): biological pathogenic factors; Cluster 2 (green): immune regulation; Cluster 3 (orange): mechanism research; Cluster 4 (dark blue): susceptibility factor research; Cluster 5 (light blue): pathological processes and molecular correlations; Cluster 6 (yellow): pathological characteristics; Cluster 7 (purple): inflammatory response, (D) timeline view of keywords related to macrophages associated periodontitis.
Keyword co-occurrence analysis is of great significance in bibliometrics. Through keyword analysis, it can reveal the theme and hot topics of the article, as well as discover the connections between keywords, which plays a crucial role in exploring research progress. We set the minimum co-occurrence value for keywords to 5 and filtered out 518 keywords that met the criteria from 4760 keywords. As shown in Figure 6C, it is mainly divided into 7 clusters. Cluster 1 (in red): biological pathogenic factors, with the main keywords being porphyromonas gingivalis, epithelial cells, and endothelial cells; Cluster 2 (green): immune regulation, with the main keywords being periodontitis, protein, and immunity; Cluster 3 (orange): mechanism research, with the main keywords being lipopolysaccharide, interleukin-6, carbon monoxide; Cluster 4 (dark blue): research on susceptibility factors, with the main keywords being inflation, infection, and diabetes; Cluster 5 (light blue): pathological processes and molecular correlations, with the main keywords being expression, bacteria, and chemotherapy; Cluster 6 (yellow): pathological characteristics, with the main keywords being cells, bone resorption, alveolar bone loss; Cluster 7 (purple): inflammatory response, with the main keywords being macrophage, matrix metalloproteins, and resveratrol. These results demonstrate the most prominent research topics and directions related to macrophages associated periodontitis to date.
In addition, we conducted a keyword timeline analysis and plotted a keyword timeline, as shown in Figure 6D. The results include “immediate response” (Cluster0), “expression” (Cluster3), “appropriate periodontitis” (Cluster4), “bone resolution” (Cluster5), “tumor crossing factor” (Cluster6), “nlrp3 inflammasome” (Cluster7), and “periodic disease” (Cluster8) “Interleukin 1 beta” (Cluster9) was a research hotspot in the early stages (2004–2010), “bone resolution” (Cluster5) was a research hotspot in the middle stages (2005–2015), and “photographic therapy” (Cluster1) and “nlrp3 inflammsome” (Cluster7) represent the most recent research hotspots in the field of macrophage associated periodontitis.
4. Discussion
In the past decade, considerable advancements have been achieved in the study of macrophages associated periodontitis. Our aim is to conduct a systematic and comprehensive analysis of the development of this field through visual analysis, and provide references for future development through keywords, hotspots, and trends.
4.1. Development overview
The analysis of 1429 papers published between 2004 and 2023 shows that the quantity of publications pertaining to macrophages associated periodontitis, as well as relative research interest, have shown a stable growth trend, particularly over the last 2 years, during which there has been a meteoric rise in the quantity of publications, indicating an increasing popularity in this field. With regard to regional and national contributions, China has the most articles contributed (419 articles, 29.32%), followed by the United States (339 articles, 23.72%), Japan (179 articles, 12.53%), South Korea (131 articles, 9.17%), and Brazil (107 articles, 7.49%). The top 5 countries contribute 82.32% of the total publication volume and are the main force of publication. Interestingly, these 5 highly productive countries still occupy the top 5 most frequently cited publications, reflecting their strong influence in the academic community. The United States has the highest amount of corresponding authors in articles originating from multiple nations, with the highest article output and strong collaborative connections with Europe and the eastern part of Asia, demonstrating that the United States is at the forefront of research in the domain of macrophage associated periodontitis. China publication volume has significantly increased in the past 3 years and may surpass the United States to become the country with the highest publication volume in the future. However, it has the largest number of corresponding authors for publications in a single country, so its level of collaboration with multiple countries is not high, and it may still not be able to catch up with the United States in the short term. At the institutional level, Sichuan University in China has the highest output (53 papers), followed by the University of São Paulo in Brazil (42 papers), and Pusan University in South Korea (40 papers), ranking third. They represent the most active research institutions, and they are mostly located in the top 5 countries with the highest productivity, indicating that the activity of research institutions can effectively improve a country’s research level. In the cooperation of scientific research institutions, South Korea and the United States have shown active performance, while China, as the country with the second highest contribution, has relatively less cooperation with its scientific research institutions. Therefore, it is necessary to cooperate with as many active institutions as possible to promote the development of this research field.
In terms of journals, the journal with the highest number of publications is Journal of Periodontal Research (98 articles), followed by Journal of Periodontology (95 articles), and Journal of Dental Research (55 articles), ranking third. Interestingly, the top 3 journals in terms of publication volume still have the highest number of citations, indicating their popularity among scholars of macrophage associated periodontitis. According to the co-citation analysis based on VOSviewer, it can be concluded that Journal of Periodontology, followed by Journal of Immunology and Journal of Clinical Periodontology, has significant influence in this field. Research from molecular/biological/genetic journals is mainly cited in molecular/biological/immunology and Dentistry/Dermatology/Surgery research. In terms of author productivity, Grenier, Daniel from University Laval have the highest productivity, while Van Dyke, T.E. have the highest H-index. Furthermore, based on VOSviewer’s co-citation analysis, it can be concluded that Hajishengallis, George N have the highest influence in the field of macrophage associated periodontitis research. At the level of cooperation among authors, authors from the same country have closer cooperation, while international author cooperation needs to be strengthened.
The most frequently cited literature in citation and co-citation analysis is Inflammation and Bone Loss in Periodontal Disease (479 times), which explains how inflammation and bone loss are markers of periodontal disease and how the former leads to the latter. Among the top 10 articles with the most citations, most of the literature types are Review Articles, which mainly describe the pathology, pathogenesis, and role of macrophage polarization in chronic inflammation of periodontitis. Our literature co citation analysis based on Citespace reveals that Hajishengallis, George publication titled Periodontitis: from microbial immune subversion to systemic inflammation has the highest citation frequency, indicating its outstanding contribution. In the realm of research pertaining to macrophage associated periodontitis. It is interesting that this article still has the highest intensity among the top 25 articles with the highest citation frequency, indicating that it continues to be a research hotspot in this field. In addition, most of the articles focus on the pathophysiology and treatment of periodontitis, as well as the expression of macrophages in periodontitis, indicating that these directions are related to macrophage associated periodontitis hot topics.
4.2. Keywords and frontiers
The burst and co-occurrence analysis of keywords reflect the development trends and hotspots of research on macrophage associated periodontitis. The word with the highest intensity is “macrophage polarization,” representing the core involvement of this keyword in periodontitis research. Li Na Zhou et al proposed that the initiation and advancement of inflammation-induced tissue damage may be attributed to the polarization of macrophages in gingival tissue,[25] and therefore, modulating macrophage function could represent a viable therapeutic approach for periodontal disease.[26,27] The keyword co-occurrence analysis based on VOSviewer showed 7 main trends, which can be divided into 7 categories: Cluster 1 (red): biological pathogenic factors; Cluster 2 (green): immune regulation; Cluster 3 (orange): mechanism; Cluster 4 (dark blue): susceptibility factors; Cluster 5 (light blue): pathological processes and molecular correlations; Cluster 6 (yellow): pathological characteristics; Cluster 7 (purple): inflammatory response. These results are in line with the promising research hotspots in the field of macrophages and osteoarthritis, as follows:
Biological pathogenic factors: keyword collinearity analysis: the key words are porphyromonas gingivalis, epiphyteal cells, and endotropical cells. Periodontitis is a chronic inflammatory illness that arises from multiple factors and is characterized by the presence of dental plaque, also known as dental biofilm/biofilm.[28] Porphyromonas gingivalis, a Gram-negative anaerobic bacterium, is often regarded as the primary pathogen responsible for the onset and progression of periodontitis.[29] The interface between the sulcus edge and coronal edge, which establishes a connection with the gingival epithelium, is in intimate proximity to bacteria residing within the gingival sulcus. This particular region plays a pivotal role in the pathogenesis of periodontal disease. P gingivalis is capable of adhering to gingival epithelial cells. The adhesion between gingival porphyrin and epithelial cells is multimodal, involving pili, proteases, hemagglutinin, and LPS, among other extracellular components and cell surfaces.[30,31] Among the numerous virulence factors produced by gingival porphyrin, fimbriae and cysteine protease attach to oral epithelial cells through different receptors and invade, especially in the initial stage of infection.[32,33] Studies have shown that Endothelial cell receptors are capable of detecting periodontal pathogens and cytokines, which are detrimental stimuli, leading to endothelial dysfunction[34] and an increased risk of cardiovascular disease in patients.[35,36]
Immunoregulation: periodontal tissue is subject to various stimuli, including oral microbiota. There is a subtle balance between local immune response and microbiota. However, after the colonization of “key” pathogens, the immune response is overactivated, leading to immune cell infiltration and activation of osteoclast activity, ultimately leading to damage to soft and hard tissues. In the early stage of pathological changes, neutrophils increase in connective tissue,[37] and macrophages,[38] lymphocytes, plasma cells, and mast cells appear.[39] Activation of complement proteins occurs.[40] Activation of the complement cascade can occur via 3 distinct pathways: the classical pathway; the lectin pathway; the alternative pathway.[41] Interestingly, despite the formation of pathogen specific antibodies in chronic periodontitis, most complement activation in this disease still occurs through classic and alternative pathways.[42] The activation of an alternative pathway is initiated by bacterial polysaccharides, including enzymes, lipopolysaccharides, or aggregated IgA. These polysaccharides facilitate the cleavage of C3 through the involvement of factor P, also known as properdin. C3b, together with factors B and D, catalyzes the conversion of C5 into its 2 components, C5a and C5b. This enzymatic cascade proceeds until the process is fully accomplished.[40]
Mechanism research: through their interaction with immune cells and tissue cells, numerous inflammatory cytokines induce periodontitis. LPS are bacterial endotoxins that can induce infiltration of polymorphonuclear leukocytes, leading to inflammatory periodontal tissue edema and vascular dilation.[43] The generation of pro-inflammatory molecules and the magnitude of inflammatory responses are influenced by the expression level of TLRs (toll-like receptors) in host cells, which can be modulated in response to LPS stimulation. Inappropriate immune responses produce excessive cytokines, accelerating the destruction of periodontal tissue.[44,45] It is known that inflammatory mediators, such as IL-6, cyclooxygenase, and intercellular adhesion molecules, has the ability to modulate the host’s immune response towards bacterial infections. The compound CORM-3 demonstrates the ability to hinder the process of macrophage polarization towards the pro-inflammatory M1 phenotype, while simultaneously facilitating the promotion of the anti-inflammatory M2 phenotype.[46] In periodontitis, CORM-3 impedes the expression of factors associated with M1 macrophages but enhances the expression of factors related to M2 macrophages.[47] By modulating macrophage polarization, CORM-3 potentially activates the NF-κB signaling pathway, thereby eliciting anti-inflammatory responses,[47,48] and other studies have shown that CORM-3 significantly enhances the osteogenic differentiation of healthy periodontal ligament stem cells (hPDLSCs) by releasing CO,[49] mediates the prospective periodontal treatment application prospects of CORM-348.
Susceptibility factor study: diabetes comprises a collection of persistent metabolic disorders distinguished by aberrant glucose metabolism, which is caused by impaired insulin secretion, insulin function, or both.[50] At present, diabetes is regarded as an unquestionable risk factor for periodontitis, contributing to the disease’s increased incidence, severity, and progression.[51] Periodontitis-induced systemic inflammation has the potential to worsen metabolic regulation, raise insulin resistance, and, over time, accelerate the onset of diabetic complications.[52] Studies have shown that patients with type 1 diabetes have higher levels of PGE2 and IL-1β in gingival sulcus fluid compared with nondiabetic patients with the same levels of periodontal disease,[53] and when challenged by lipopolysaccharides, monocytes from patients with type 1 diabetes produced significantly higher concentrations of TNF-α, IL-1β, and PGE2 than monocytes from non-diabetic individual.[54] Therefore, it can be stated that the excessive inflammatory response and damaged repair of bacterial attack are possible explanations for the link between periodontitis and diabetes. To fully understand how periodontal infection influences the state of diabetes, more research is required. In addition, there is an established relationship between periodontitis and atherosclerotic vascular disease (the main cause of cardiovascular disease).[55] Several mechanisms have been proposed to explain this very close relationship, in an animal dosing study of GroEL, GroEL increased the expression of ICAM-1, VCAM-1, LOX-1, and TLR4 in the aorta of wild C57BL/6 mice, but not in C57BL/6-Tlr4lps-del mice. Indicates gingival p. GroEL may contribute to cardiovascular disease by affecting the expression of TLR4,[56] Porphyromonas gingivitis infection alters the vascular reactivity of spontaneous atherosclerosis compared with wild-type mice with apolipoprotein e deficiency C57BL/6 (C57) mice,[57] induces changes in systemic cytokine levels (selectively up-regulated by MMP3, ICAM-1, IGFBP-2, and chemokine CXCL7, and selectively down-regulated by IL-17 and TNF-a), which play a role not only in periodontal tissue destruction, but also in the development of atherosclerosis.[58]
Molecular related research: the interaction between pro-inflammatory and/or anti-inflammatory cytokines in patients with periodontitis is related to the pathogenesis of the disease. The overexpression of pro-inflammatory cytokines, such as TNF-α, IL-1 β, IL-17, IL-6, and chemokines CXCL-6 and CXCL-8,[59] is linked to the advancement of periodontitis patients. However, levels of the anti-inflammatory IL-4 and IL-11, as well as IFN and IL-12, also reduced. This suggests that γ expression has a dual role in periodontal disease.[60]
Pathological characteristics: the main pathological manifestations of periodontitis are the formation of periodontal pockets and chronic inflammation of the pocket wall, loss of attachment, and alveolar bone resorption.[61] Periodontitis usually occurs when gingivitis is not adequately treated, and there are often large amounts of plaque and stones (food scraps, saliva, germs, and mucus-containing stones that include calcium and phosphate) located below the gum edge. In periodontitis, deeper pockets are formed in the periodontal tissue and can accommodate anaerobic microorganisms, which pose greater harm than basic gingivitis. Colonizing bacteria include Actinobacteria actinomycetes, P gingivalis, Enterococcus aeruginosa, and many Gram negative bacteria. These microorganisms induce neutrophils and monocytes to continuously secrete inflammatory mediators, such as cytokines, prostaglandins, and enzymes. Inflammation impacts the periodontal ligament, alveolar bone, cementum, and gums. Progressive loss of gum attachment to teeth, deepening of periodontal pockets, absorption of alveolar bone with progressive bone loss, loosening of teeth, and gum recession. Later stages frequently involve tooth migration, which can lead to the eventual loss of a tooth.
Inflammatory responses: macrophages can be classified into 2 distinct phenotypes: pro-inflammatory macrophages, also known as M1 macrophages, and anti-inflammatory or regulatory macrophages, referred to as M2 macrophages. M1 macrophages are characterized by their association with interleukin-12 and IL-8, which facilitate the activation of type 1 Th1 cells. This activation promotes the pro-inflammatory phase of the immune response. On the other hand, M2 macrophages are associated with TGF-β, vascular endothelial growth factor, or EGF. These factors contribute to the activation of Th2 cells, which in turn promote the healing and regression phases.[38,62] The polymorphism of MMPs genes is associated with periodontal disease risk. The MMP-9-753 C/T polymorphism, for instance, decreases the risk of developing chronic periodontitis, whereas the MMP-3-1171 5A/6A and MMP-8-799 C/T polymorphisms augment the risk of developing chronic periodontitis.[62] P gingivitis may also increase monocyte migration by activating the expression of MMP-9,[63] which indirectly leads to tissue damage. However, the complexity of host microbial interactions in periodontal tissue has not yet been elucidated. Research has demonstrated that resveratrol safeguards rat periodontal tissue against injury through the inhibition of inflammatory responses and the stimulation of antioxidant defense mechanisms.[64] In a rat model of periodontitis, this compound also activates the nuclear factor erythroid 2 related factor 2 (Nrf2) pathway, reducing oxidative stress and pro-inflammatory cytokine production.[65] In addition, resveratrol blocks the NF-κB signaling cascade, which in turn prevents human gingival epithelial cells from responding inflammatory ally.[66] Furthermore, even with long-term use, no toxic effects of this compound have been found.[67] These findings indicate that resveratrol, with its lower side effects, enhances alveolar bone loss by suppressing periodontal tissue inflammatory response and upregulating the expression of redox-sensitive molecules in cells. It may become one of the drugs for treating periodontitis in the future.
The keyword timeline chart shows the evolution of keywords over time, which reflects that there are many early hotspots and their frequency is high. In the past 2 years, “extra cellar vessel” and “autophagy” have become the latest research hotspots. Studies have shown that there are differences in the expression of plasma derived exosomes miRs (miR1304-3p and miR-200c-3p) and snoRs (SNORD57 and SNODB1771) in patients with periodontitis,[68] which may be helpful in diagnosing. In addition, extracellular vesicles can also participate in the treatment of periodontitis. Exos from 3D cultured MSCs not only restored Th17 cell/Treg balance through miR-1246/Nfat5 axis, but also restored immune response in inflamed periodontal tissue.[69] Dental pulp stem cells exos can promote the transformation of macrophages from pro-inflammatory phenotype (M1) to anti-inflammatory phenotype (M2), promoting alveolar bone healing in periodontitis mice.[70] Exos secreted by hPDLSCs promote osteogenic differentiation of PDLSCs from periodontitis tissue.[71] Therefore, the treatment strategy for extracellular periodontitis has the potential to overcome the shortcomings of traditional treatment methods and has great clinical application prospects. Autophagy is a natural regulatory mechanism for the degradation of damaged organelles and the circulation of cytoplasmic substances, involving various pathophysiology. A study has found that the levels of LC3b positive cells and LC3-II protein in gingival tissue of patients with periodontitis are higher than those in healthy gingival tissue,[72] indicating an increase in autophagy in gingival tissue of patients with periodontitis. In addition, autophagy induced by periodontal pathogens invasion enhances the survival ability of pathogens by bypassing the immune system, and aids in the destruction of intracellular microorganisms through antibacterial processes. The effect of autophagy on periodontitis-related pro-inflammatory cytokine production has not been determined. Studies have shown that the Akt/mTOR pathway is a critical medium in signaling Transduction pathways that may be associated with autophagy process,[73] P gingivitis induced AKT/mTOR signaling prevents human dendritic cells from going through the antimicrobial autophagy process, which allows P gingivalis to survive intracellularly.[74] Hence, the mechanism by which its function is transduced may serve as a potential target for novel periodontal treatments.
Macrophages play a pivotal role in the field of periodontology, particularly in orthodontic treatments and the complex interplay between pulp and periodontal tissues. During the initial phase of orthodontic therapy, mechanical forces applied to teeth lead to the activation of local macrophages, which produce inflammatory mediators such as IL-1β.[75] This early inflammatory response is crucial for the initial stages of tooth movement, as IL-1β not only promotes bone resorption but also influences the velocity and efficiency of dental movement. Beyond their role in initiating the inflammatory process, macrophages secrete cytokines like macrophage colony-stimulating factor, which affect the activity of osteoblasts and osteoclasts, thereby regulating the processes of bone resorption and new bone formation. Macrophage colony-stimulating factor is particularly important for the recruitment and differentiation of early osteoclasts, which is a significant factor in tooth movement.[76] Macrophages also contribute to angiogenesis by secreting vascular endothelial growth factor, which promotes the formation of new blood vessels. This process is essential for the remodeling of the periodontal ligament and the resorption and formation of bone tissue. In the context of pulp inflammation, macrophages recognize and respond to bacterial products, activating an inflammatory response and releasing cytokines such as TNF-α and IL-1β. These cytokines play a critical role in the inflammatory process. However, macrophages also participate in the resolution of inflammation by polarizing to the M2 phenotype, which reduces inflammation and promotes tissue repair.[77]
4.3. Future research trends
Predicting the future trend of macrophage associated periodontitis is of great significance. The future research hotspots tend to focus on macrophage polarization, mechanism research, nlpr3 inflammasome, and nanoparticle, indicating that the molecular biology exploration of periodontitis has moved in another direction. The progression of periodontitis and the promotion of periodontal tissue recovery can be controlled by targeting macrophages to M1 or M2 through regulatory factors. Specific inhibitors such as nlpr3 inflammasome can also be used to inhibit osteoclast differentiation and alleviate bone loss. Alternatively, through nanotechnology, specific receptors or targets can be combined to act on macrophages or pathogens, in order to halt disease progression or promote healing. Moreover, it has the potential to stimulate the process of stem cell differentiation, activate their osteogenic ability, and promote the healing of periodontal tissue to achieve functional recovery.
4.4. Limitation
Due to differences in research update speed and uncertainty in publication status, there may be omissions in existing data. In addition, as we only collected information from the WoSCC database and did not include information from databases such as PubMed and Cochrane, this may lead to biases in the analysis and prediction results. Therefore, in future research, more data sources and more scientific bibliometric analysis methods are needed to achieve more accurate and effective research results.
5. Conclusion
In summary, this work represents the first thorough and rigorous bibliometric review of the previous 20 years’ global research trends in macrophage-associated periodontitis. This study systematically summarizes and analyzes the current research status, and analyzes and visualizes the country and institutions, journals and authors, citations and keywords. We have made speculations and reasonable suggestions for the future development and collaboration of countries, institutions, journals, and authors based on the existing research status. And based on the current main research hotspots, reasonably infer the research directions that may continue or appear in the future.
Acknowledgments
We would like to appreciate the Affiliated Hospital of Stomatology of Anhui Medical University for its support and help in this study.
Author contributions
Data curation: Hu Zheng, Wenjia Han.
Formal analysis: Hu Zheng, Keyi Liu.
Funding acquisition: Yuanyin Wang, Ran Chen.
Investigation: Yuhang Cai, Wenjia Han.
Methodology: Keyi Liu, Wenjia Han.
Software: Junwei Xiang.
Supervision: Hu Zheng.
Validation: Yuhang Cai.
Writing – original draft: Hu Zheng.
Writing – review & editing: Yuanyin Wang, Ran Chen.
Supplementary Material
Abbreviations:
- hPDLSCs
- healthy periodontal ligament stem cells
- IF
- Impact Factor
- LPS
- lipopolysaccharide
- TLRs
- toll-like receptors
- WoSCC
- Web of Science Core Collection
This article is a bibliometric study and does not cover any ethical aspects, so it does not require ethical approval.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental Digital Content is available for this article.
How to cite this article: Zheng H, Cai Y, Liu K, Xiang J, Han W, Wang Y, Chen R. Visualize the time dynamics and research trends of macrophage associated periodontitis research from 2004 to 2023: Bibliometrix analysis. Medicine 2024;103:46(e40450).
HZ and YC contributed equally to this work.
Contributor Information
Hu Zheng, Email: zhenghu989898@163.com.
Yuhang Cai, Email: c13620001022@163.com.
Keyi Liu, Email: m18955771764@163.com.
Junwei Xiang, Email: xjw_1900@163.com.
Wenjia Han, Email: 18055792108@163.com.
Yuanyin Wang, Email: Wyy1970548@sohu.com.
References
- [1].Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Luo LS, Luan HH, Jiang JF, et al. The spatial and temporal trends of severe periodontitis burden in Asia, 1990–2019: a population-based epidemiological study. J Periodontol. 2022;93:1615–25. [DOI] [PubMed] [Google Scholar]
- [3].Tonetti MS, Jepsen S, Jin L, Otomo Corgel J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: a call for global action. J Clin Periodontol. 2017;44:456–62. [DOI] [PubMed] [Google Scholar]
- [4].Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Clin Periodontol. 2018;45:419–42. [DOI] [PubMed] [Google Scholar]
- [5].Teles R, Moss K, Preisser JS, et al. Patterns of periodontal disease progression based on linear mixed models of clinical attachment loss. J Clin Periodontol. 2018;45:15–25. [DOI] [PubMed] [Google Scholar]
- [6].Zhu J, Chu W, Luo J, Yang J, He L, Li J. Dental materials for oral microbiota dysbiosis: an update. Front Cell Infect Microbiol. 2022;12:808349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Meyle J, Chapple IL. Molecular aspects of the pathogenesis of periodontitis. Periodontol 2000. 2015;69:7–17. [DOI] [PubMed] [Google Scholar]
- [8].Netea MG, Balkwill F, Chonchol M, et al. Author correction: a guiding map for inflammation. Nat Immunol. 2021;22:254. [DOI] [PubMed] [Google Scholar]
- [9].Nestle FO, Thompson C, Shimizu Y, Turka LA, Nickoloff BJ. Costimulation of superantigen-activated T lymphocytes by autologous dendritic cells is dependent on B7. Cell Immunol. 1994;156:220–9. [DOI] [PubMed] [Google Scholar]
- [10].Niu L, Chen S, Yang X, et al. Vitamin D decreases Porphyromonas gingivalis internalized into macrophages by promoting autophagy. Oral Dis. 2021;27:1775–88. [DOI] [PubMed] [Google Scholar]
- [11].Gardai SJ, McPhillips KA, Frasch SC, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–34. [DOI] [PubMed] [Google Scholar]
- [12].Becerra Ruiz JS, Guerrero Velázquez C, Martínez Esquivias F, Martínez Pérez LA, Guzmán Flores JM. Innate and adaptive immunity of periodontal disease. from etiology to alveolar bone loss. Oral Dis. 2022;28:1441–7. [DOI] [PubMed] [Google Scholar]
- [13].Ushach I, Zlotnik A. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J Leukoc Biol. 2016;100:481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wei W, Zhang Y, Song Q, et al. Transmissible ER stress between macrophages and tumor cells configures tumor microenvironment. Cell Mol Life Sci. 2022;79:1687–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang X, Du H, Li X. Artesunate attenuates atherosclerosis by inhibiting macrophage M1-like polarization and improving metabolism. Int Immunopharmacol. 2022;102:108413. [DOI] [PubMed] [Google Scholar]
- [16].Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Orihuela R, McPherson CA, Harry GJ. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol. 2016;173:649–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hu K, Shang Z, Yang X, Zhang Y, Cao L. Macrophage polarization and the regulation of bone immunity in bone homeostasis. J Inflamm Res. 2023;16:3563–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090. [DOI] [PubMed] [Google Scholar]
- [20].Kang S, Nakanishi Y, Kioi Y, et al. Semaphorin 6D reverse signaling controls macrophage lipid metabolism and anti-inflammatory polarization. Nat Immunol. 2018;19:561–70. [DOI] [PubMed] [Google Scholar]
- [21].Cavalla F, Hernández M. Polarization Profiles of T Lymphocytes and Macrophages Responses in Periodontitis. Cham: Springer International Publishing; 2022:195–208. [DOI] [PubMed] [Google Scholar]
- [22].Ortega-Gómez A, Perretti M, Soehnlein O. Keywords: apoptosis; efferocytosis; macrophage reprogramming; pro-resolving drugs; tissue homeostasis. EMBO Mol Med. 2013;5:661–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kong L, Wang Y, Smith W, Hao AD. Macrophages in bone homeostasis. Curr Stem Cell Res Ther. 2019;6:474–81. [DOI] [PubMed] [Google Scholar]
- [24].Ahmad P, Slots J. A bibliometric analysis of periodontology. Periodontol 2000. 2021;85:237–40. [DOI] [PubMed] [Google Scholar]
- [25].Zhou LN, Bi CS, Gao LN, An Y, Chen F, Chen FM. Macrophage polarization in human gingival tissue in response to periodontal disease. Oral Dis. 2019;25:265–73. [DOI] [PubMed] [Google Scholar]
- [26].Sharma M, Patterson L, Chapman E, Flood PM. Salmeterol, a long-acting β2-adrenergic receptor agonist, inhibits macrophage activation by lipopolysaccharide from Porphyromonas gingivalis. J Periodontol. 2017;88:681–92. [DOI] [PubMed] [Google Scholar]
- [27].Yu T, Zhao L, Huang X, et al. Enhanced activity of the macrophage M1/M2 phenotypes and phenotypic switch to M1 in periodontal infection. J Periodontol. 2016;87:1092–102. [DOI] [PubMed] [Google Scholar]
- [28].Spahr A, Divnic Resnik T. Impact of health and lifestyle food supplements on periodontal tissues and health. Periodontol 2000. 2022;90:146–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Alshehri FA, Alharbi MS. The effect of adjunctive use of hyaluronic acid on prevalence of Porphyromonas gingivalis in subgingival biofilm in patients with chronic periodontitis: a systematic review. Pharmaceutics. 2023;15:1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Takeuchi H, Sasaki N, Yamaga S, Kuboniwa M, Matsusaki M, Amano A. Porphyromonas gingivalis induces penetration of lipopolysaccharide and peptidoglycan through the gingival epithelium via degradation of junctional adhesion molecule 1. PLoS Pathog. 2019;15:e1008124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shoji M, Shibata S, Sueyoshi T, Naito M, Nakayama K. Biogenesis of type V pili. Microbiol Immunol. 2020;64:643–56. [DOI] [PubMed] [Google Scholar]
- [32].Chen T, Nakayama K, Belliveau L, Duncan M. Porphyromonas gingivalis gingipains and adhesion to epithelial cells. Infect Immun. 2001;5:3048–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Andrian E, Grenier D, Rouabhia M. Porphyromonas gingivalis-epithelial cell interactions in periodontitis. J Dent Res. 2006;85:392–403. [DOI] [PubMed] [Google Scholar]
- [34].Xie M, Tang Q, Yu S, et al. Porphyromonas gingivalis disrupts vascular endothelial homeostasis in a TLR-NF-κB axis dependent manner. Int J Oral Sci. 2020;12:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Roth GA, Moser B, Roth-Walter F, et al. Infection with a periodontal pathogen increases mononuclear cell adhesion to human aortic endothelial cells. Atherosclerosis. 2007;190:271–81. [DOI] [PubMed] [Google Scholar]
- [36].Sun W, Wu J, Lin L, Huang Y, Chen Q, Ji Y. Porphyromonas gingivitis stimulates the release of nitric oxide by inducing expression of inducible nitric oxide synthases and inhibiting endothelial nitric oxide synthases. J Periodontal Res. 2010;45:381–8. [DOI] [PubMed] [Google Scholar]
- [37].Nicu EA, Loos BG. Polymorphonuclear neutrophils in periodontitis and their possible modulation as a therapeutic approach. Periodontol 2000. 2016;71:140–63. [DOI] [PubMed] [Google Scholar]
- [38].Sun X, Gao J, Meng X, Lu X, Zhang L, Chen R. Polarized macrophages in periodontitis: characteristics, function, and molecular signaling. Front Immunol. 2021;12:763334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Silva TA, Garlet GP, Fukada SY, Silva JS, Cunha FQ. Chemokines in oral inflammatory diseases: apical periodontitis and periodontal disease. J Dent Res. 2007;86:306–19. [DOI] [PubMed] [Google Scholar]
- [40].Hajishengallis G. Complement and periodontitis. Biochem Pharmacol. 2010;80:1992–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dunkelberger JR, Song W. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20:34–50. [DOI] [PubMed] [Google Scholar]
- [42].Navarrete M, García J, Dutzan N, et al. Interferon-γ, interleukins-6 and -4, and factor XIII-A as indirect markers of the classical and alternative macrophage activation pathways in chronic periodontitis. J Periodontol. 2014;85:751–60. [DOI] [PubMed] [Google Scholar]
- [43].Duan Y, An W, Wu Y, Wang J. Tetramethylpyrazine reduces inflammation levels and the apoptosis of LPS-stimulated human periodontal ligament cells via the downregulation of miR-302b. Int J Mol Med. 2020;45:1918–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20. [DOI] [PubMed] [Google Scholar]
- [45].Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2004;17:1–14. [DOI] [PubMed] [Google Scholar]
- [46].Yamamoto-Oka H, Mizuguchi S, Toda M, et al. Carbon monoxide-releasing molecule, CORM-3, modulates alveolar macrophage M1/M2 phenotype in vitro. Inflammopharmacology. 2018;26:435–45. [DOI] [PubMed] [Google Scholar]
- [47].Liu T, Han Q, Pan Y, Li J, Song H. Carbon monoxide-releasing molecule-3 regulates the polarization of lipopolysaccharide-induced macrophages. Inflammation. 2021;44:1737–49. [DOI] [PubMed] [Google Scholar]
- [48].Choi E, Choe S, Hyeon J, Choi J, Choi IS, Kim S. Carbon monoxide-releasing molecule-3 suppresses Prevotella intermedia lipopolysaccharide-induced production of nitric oxide and interleukin-1β in murine macrophages. Eur J Pharmacol. 2015;764:22–9. [DOI] [PubMed] [Google Scholar]
- [49].Chen H, Dai Y, Cui J, et al. Carbon monoxide releasing molecule-3 enhances osteogenic differentiation of human periodontal ligament stem cells by carbon monoxide release. Drug Des Devel Ther. 2021;15:1691–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Klatka M, Rysz I, Hymos A, et al. Effect of Epstein-Barr virus infection on selected immunological parameters in children with type 1 diabetes. Int J Mol Sci. 2023;24:2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Preshaw PM, Alba AL, Herrera D, et al. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. 2011;7:738–48. [DOI] [PubMed] [Google Scholar]
- [53].Salvi GE, Yaida B, Collins JG, et al. Inflammatory mediator response as a potential risk marker for periodontal diseases in insulin-dependent diabetes mellitus patients. J Periodontol. 1997;68:127–35. [DOI] [PubMed] [Google Scholar]
- [54].Salvi GE, Collins JG, Yalda B, Arnold RR, Lang NP, Offenbacher S. Monocytic tumor necrosis factor alpha secretion patterns in insulin-dependent diabetes mellitus patients with periodontal diseases. J Clin Periodontol. 1997;24:8–16. [DOI] [PubMed] [Google Scholar]
- [55].Schenkein HA, Papapanou PN, Genco R, Sanz M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontol 2000. 2020;83:90–106. [DOI] [PubMed] [Google Scholar]
- [56].Huang C, Shih C, Tsao N, et al. Original article: the GroEL protein of Porphyromonas gingivalis regulates atherogenic phenomena in endothelial cells mediated by upregulating toll-like receptor 4 expression. Am J Transl Res. 2016;2:384–404. [PMC free article] [PubMed] [Google Scholar]
- [57].Pereira RB, Vasquez EC, Stefanon I, Meyrelles SS. Oral P. gingivalis infection alters the vascular reactivity in healthy and spontaneously atherosclerotic mice. Lipids Health Dis. 2011;10:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Miyauchi S, Maekawa T, Aoki Y, et al. Oral infection with Porphyromonas gingivalis and systemic cytokine profile in C57BL/6.KOR-ApoEshl mice. J Periodontal Res. 2012;47:402–8. [DOI] [PubMed] [Google Scholar]
- [59].Tripathy AS, Vishwakarma S, Trimbake D, et al. Pro-inflammatory CXCL-10, TNF-α, IL-1β, and IL-6: biomarkers of SARS-CoV-2 infection. Arch Virol. 2021;166:3301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Pan W, Wang Q, Chen Q. The cytokine network involved in the host immune response to periodontitis. Int J Oral Sci. 2019;11:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Amato M, Santonocito S, Viglianisi G, Tatullo M, Isola G. Impact of oral mesenchymal stem cells applications as a promising therapeutic target in the therapy of periodontal disease. Int J Mol Sci. 2022;23:13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Weng H, Yan Y, Jin Y, Meng X, Mo Y, Zeng X. Matrix metalloproteinase gene polymorphisms and periodontitis susceptibility: a meta-analysis involving 6162 individuals. Sci Rep. 2016;6:26290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zhou J, Zhang J, Chao J. Porphyromonas gingivitis promotes monocyte migration by activating MMP-9. J Periodontal Res. 2012;47:236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Bhattarai G, Poudel SB, Kook S, Lee J. Resveratrol prevents alveolar bone loss in an experimental rat model of periodontitis. Acta Biomater. 2016;29:398–408. [DOI] [PubMed] [Google Scholar]
- [65].Tamaki N, Cristina Orihuela-Campos R, Inagaki Y, Fukui M, Nagata T, Ito H. Resveratrol improves oxidative stress and prevents the progression of periodontitis via the activation of the Sirt1/AMPK and the Nrf2/antioxidant defense pathways in a rat periodontitis model. Free Radic Biol Med. 2014;75:222–9. [DOI] [PubMed] [Google Scholar]
- [66].Minagawa T, Okui T, Takahashi N, et al. Resveratrol suppresses the inflammatory responses of human gingival epithelial cells in a SIRT1 independent manner. J Periodontal Res. 2015;50:586–93. [DOI] [PubMed] [Google Scholar]
- [67].Cottart CH, Nivet Antoine V, Laguillier Morizot C, Beaudeux JL. Resveratrol bioavailability and toxicity in humans. Mol Nutr Food Res. 2010;54:7–16. [DOI] [PubMed] [Google Scholar]
- [68].Lin H, Chen H, Zhao X, et al. Advances of exosomes in periodontitis treatment. J Transl Med. 2022;20:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zhang Y, Chen J, Fu H, et al. Exosomes derived from 3D-cultured mesenchymal stem cells improve therapeutic effects in periodontitis and experimental colitis and restore the Th17 cell/Treg balance in inflamed periodontium. Int J Oral Sci. 2021;13:448–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Shen Z, Kuang S, Zhang Y, et al. Chitosan hydrogel incorporated with dental pulp stem cell-derived exosomes alleviates periodontitis in mice via a macrophage-dependent mechanism. Bioact Mater. 2020;5:1113–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lei F, Li M, Lin T, Zhou H, Wang F, Su X. Treatment of inflammatory bone loss in periodontitis by stem cell-derived exosomes. Acta Biomater. 2022;141:333–43. [DOI] [PubMed] [Google Scholar]
- [72].Kim WJ, Park SY, Kim OS, Park HS, Jung JY. Autophagy upregulates inflammatory cytokines in gingival tissue of patients with periodontitis and lipopolysaccharide-stimulated human gingival fibroblasts. J Periodontol. 2022;93:380–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Hou X, Hu Z, Xu H, et al. Advanced glycation endproducts trigger autophagy in cardiomyocyte via RAGE/PI3K/AKT/mTOR pathway. Cardiovasc Diabetol. 2014;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Meghil MM, Tawfik OK, Elashiry M, et al. Disruption of immune homeostasis in human dendritic cells via regulation of autophagy and apoptosis by Porphyromonas gingivalis. Front Immunol. 2019;10:2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Alikhani M, Chou MY, Khoo E, et al. Age-dependent biologic response to orthodontic forces. Am J Orthod Dentofacial Orthop. 2018;153:632–44. [DOI] [PubMed] [Google Scholar]
- [76].Ma QL, Fang L, Jiang N, et al. Bone mesenchymal stem cell secretion of sRANKL/OPG/M-CSF in response to macrophage-mediated inflammatory response influences osteogenesis on nanostructured Ti surfaces. Biomaterials. 2018;154:234–47. [DOI] [PubMed] [Google Scholar]
- [77].Batra R, Suh MK, Carson JS, et al. IL-1β (interleukin-1β) and TNF-α (tumor necrosis factor-α) impact abdominal aortic aneurysm formation by differential effects on macrophage polarization. Arterioscler Thromb Vasc Biol. 2018;38:457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]