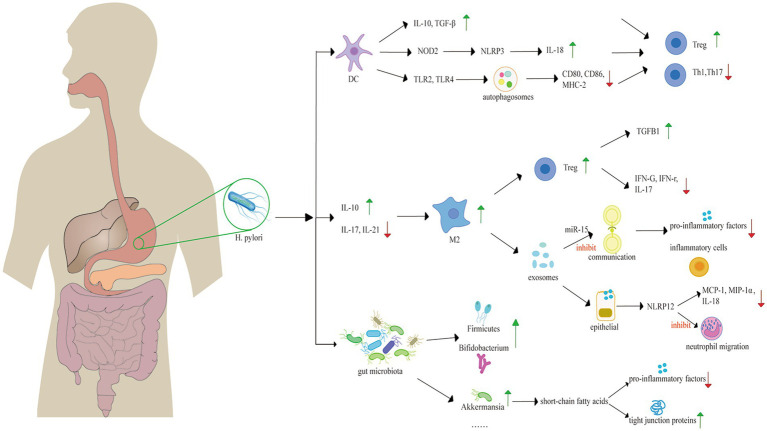
Figure 5.
Mechanisms underlying the protective role of H. pylori against IBD, including the change of gut microbiota and immune system regulation. H. pylori promotes the transformation of DCs into tolerogenic DCs, expressing high levels of anti-inflammatory factors, such as IL-10 and TGF-β. H. pylori activates NOD2 to activate the NLRP3 inflammasome of DCs, leading to the expression of IL-18. H. pylori triggers autophagosome activation, diminishing the secretion of MHC-2, CD80 and CD86. The secretion of these immune factors induce the differentiation of immunosuppressive Tregs, rather than Th1 or Th17 cells. H. pylori also induces the switch of M2 macrophages with increasing IL-10 and diminishing IL-17 and IL-21. M2 macrophages can induce the proliferation of Treg, conferring an anti-inflammatory effect. Exosomes derived from macrophages can suppress the inflammatory response. H. pylori alters gut microbiota with a healthier diversity, increasing SCFAs production, and protecting the intestinal barrier with increased tight junction proteins. H. pylori, Helicobacter pylori; DC, dendritic cell; IL, interleukin; TLR, Toll-like receptor; Treg, regulatory T cell; M2, M2 macrophage.