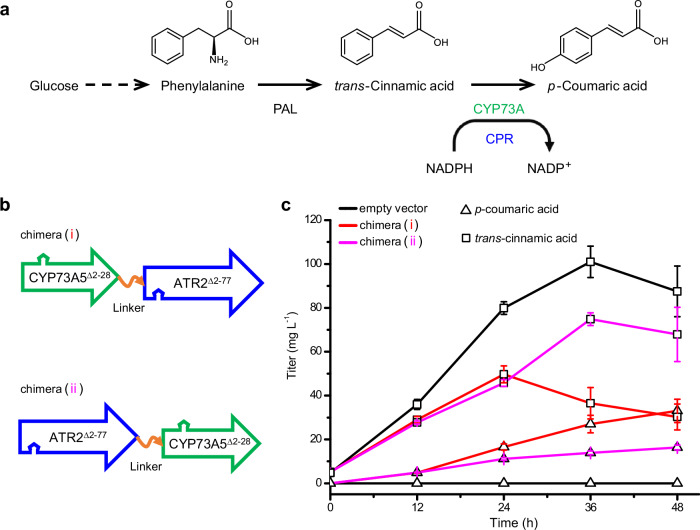
Fig. 2. Effects of the chimeric architecture on the biosynthetic performance of eukaryotic P450 enzyme.
a Plant p-coumaric acid biosynthesis, involving the plant-specific CYP73A subfamily along with CPR and the upstream phenylalanine ammonia-lyase (PAL), was targeted in prokaryotic E. coli. b Two types of tandem fused chimeras were constructed via fusing A. thaliana CYP73A5 in frame to either the N-terminus (i) or the C-terminus (ii) of A. thaliana P450 reductase 2 (ATR2) with a flexible peptide linker (L) as an inserted hinge, indicated by the orange curve with a single arrow. The N-terminal anchors of CYP73A5 (amino acid residues 2 to 28) and ATR2 (amino acid residues 2 to 77) were truncated. The arrow indicates a polypeptide from the N-terminus to the C-terminus. c p-Coumaric acid de novo biosynthesis (triangle) and trans-cinnamic acid accumulation (square) in A. thaliana PAL1-expressed E. coli strains with empty vector (black line), chimera (i) (red line), or chimera (ii) (magenta line). Data are shown as mean ± SE (n = 9 biological independent clones). P value of p-coumaric acid titer at 48 hpi was calculated by two-sided t-test (P = 0.018). Source data are provided as a Source Data file.