Abstract
Bispecific T-cell engagers (BiTEs) bring together tumour cells and cytotoxic T cells by binding to specific cell-surface tumour antigens and T-cell receptors, and have been clinically successful for the treatment of B-cell malignancies. Here we show that a BiTE–sialidase fusion protein enhances the susceptibility of solid tumours to BiTE-mediated cytolysis of tumour cells via targeted desialylation—that is, the removal of terminal sialic acid residues on glycans—at the BiTE-induced T-cell–tumour-cell interface. In xenograft and syngeneic mouse models of leukaemia and of melanoma and breast cancer, and compared with the parental BiTE molecules, targeted desialylation via the BiTE–sialidase fusion proteins enhanced the formation of immunological synapses, T-cell activation and T-cell-mediated tumour-cell cytolysis in the presence of the target antigen. The targeted desialylation of tumour cells may enhance the potency of therapies relying on T-cell engagers.
A central theme in cancer immunotherapy is the activation of a patient’s own immune system for tumour control. Bispecific T-cell engagers (BiTEs) are off-the-shelf agents that recruit endogenous CD8+ and CD4+ T cells capable of eradicating tumour cells in a manner that is independent of the major histocompatibility complex1. A BiTE molecule consists of two single-chain variable fragments (scFvs), one targeting a tumour-associated antigen and the other binding to CD3 on T cells. These two scFvs are covalently connected by a small linker peptide. Blinatumomab, which targets the CD19 antigen present on B cells, is the first BiTE approved by the US Food and Drug Administration (FDA) for the treatment of B-cell precursor acute lymphoblastic leukaemia in patients with residual cancer after chemotherapy2.
However, as with most T-cell-based therapies, the promise of BiTEs in the treatment of solid tumours has yet to be realized3. In addition to the problem of limited tumour tissue penetration, T-cell-based therapies must overcome the immunosuppressive tumour microenvironment in which T-cell suppression is orchestrated by tumour cells and the neighbouring stromal, myeloid and lymphoid cells4. In this unique microenvironment, limited nutrient availability and accumulated metabolic waste products lead to alterations in cell-surface epitopes of both tumour and immune cells, which subsequently alter their interactions and ultimately lead to T-cell dysfunction and poor tumour control5. Therefore, enabling technologies that target the molecular and cellular components of the immunosuppressive tumour microenvironment have the potential to transform T-cell-based cancer therapies, including those based on T-cell-engaging approaches.
Aberrant glycosylation is a hallmark of cancer6–9. Tumour cells often express a heavily sialylated glycocalyx as a physical barrier to prevent the effective T-cell infiltration into the tumour bed. These upregulated glycoforms with terminal sialic acid can then act as glyco-immune checkpoints to suppress immune activation10,11. Sialosides attenuate immune cell activation and effector function by recruiting sialic acid-binding immunoglobulin-like lectins (Siglecs) that are found on most leukocytes to the immunological synapse (IS), where they can trigger inhibitory signalling12,13. In addition, sialosides expressed on T cells and antigen-presenting cells (APCs) may interact with CD28 on T cells to compete with its binding to CD80 on APCs, resulting in suppression of the co-stimulation required for T-cell activation and survival14.
Previous studies have shown that selective inhibition of cell-surface sialylation in the tumour microenvironment via intratumoural administration of an unnatural sialic acid mimic, P-3Fax-Neu5Ac, which inhibits sialyltransferases, is a powerful intervention that potentiates T-cell-mediated cancer cell killing while reducing the infiltrating regulatory T cells and myeloid suppressor cells15. Sialoside blockade enhanced antigen-specific CD8+ T-cell-mediated cytolysis of tumour cells, in part by facilitating clustering of tumour cells with T cells. Inspired by this and other work demonstrating the importance of hypersialylation in suppressing T-cell-induced killing16, we report here the development of BiTE–sialidase fusion proteins that can remove sialoglycans at the T-cell–tumour-cell interface engaged by BiTE molecules, leading to an increase in T-cell-dependent tumour-cell cytolysis. We demonstrate that, in vitro, the enhanced tumour-cell cytolysis is independent of the inhibitory sialoside–Siglec signalling but results from a stronger IS formation induced by BiTE molecules. Furthermore, we show in several preclinical models of blood and solid tumours that BiTE–sialidase fusion proteins exhibit superior efficacy in controlling tumour proliferation and prolonging survival in vivo compared with the parental BiTE molecules.
Results
Removal of sialic acids on the surface of tumour cells enhances BiTE-mediated tumour-cell killing by T cells
To evaluate whether desialylation may enhance the susceptibility of tumour cells to BiTE-mediated cytotoxicity by T cells, we first constructed a BiTE molecule, 4D5 BiTE, from a high-affinity HER2-targeting scFv 4D5 derived from Herceptin (dissociation constant (KD) ≈ 5 nM) and a moderate-affinity human CD3-targeting scFv (KD ≈ 260 nM in the BiTE format, the clone TR66 based on which blinatumomab was constructed)17,18. We then treated HER2-positive SK-BR-3 human breast cancer cells with a sialidase derived from Bifidobacterium longum subsp. infantis (B. infantis) to remove cell-surface sialic acids. Expressed by human commensal bacteria B. infantis, this sialidase is known to hydrolyse both α2–3- and α2–6-linked sialosides with high efficiency19.
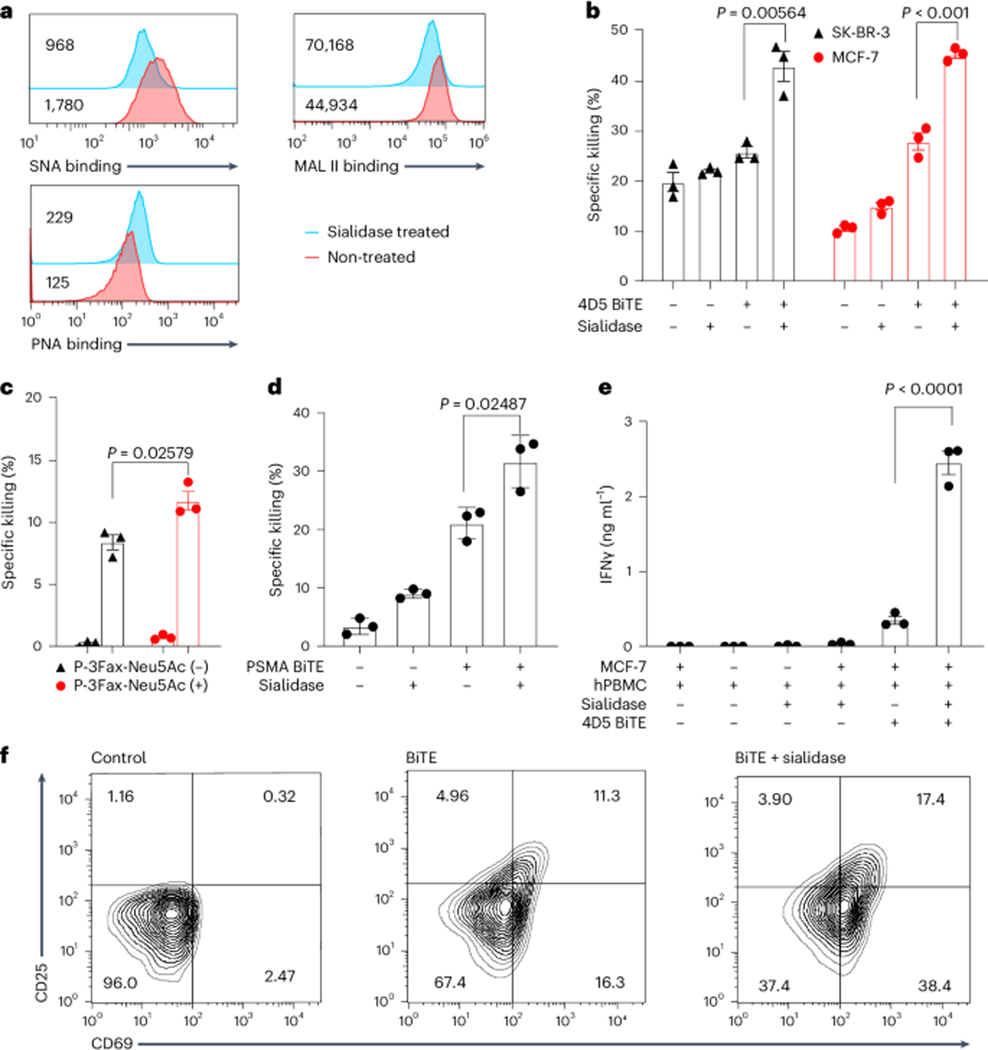
Staining with Sambucus nigra agglutinin (SNA) that binds preferentially to sialic acid attached to terminal galactose in an α2–6 linkage, Maackia amurensis lectin II (MAL II) that binds to α2–3 linkage and exposed galactose-binding peanut agglutinin (PNA) confirmed the success of cell-surface desialylation (Fig. 1a). Next, we incubated SK-BR-3 cells with 4D5 BiTE and peripheral blood mononuclear cells (PBMCs) from healthy human donors in the presence or absence of B. infantis sialidase. At different effector-to-target ratios, the addition of sialidase markedly potentiated the 4D5 BiTE-induced tumour-cell lysis by T cells compared with 4D5 BiTE alone (Fig. 1b and Supplementary Fig. 1a). Target cell killing was also enhanced by desialylation of either effector cells or target tumour cells alone (Supplementary Fig. 1b). The improvement of the BiTE-induced killing was sialidase dose dependent (Supplementary Fig. 1c). A similar trend was observed when another HER2-expressing breast cancer cell line, MCF-7, was used as the target cell and when 4D5 BiTE treatment was combined with a sialylation inhibitor, P-3Fax-Neu5Ac (Fig. 1b,c). To verify that the enhancement of BiTE-induced killing by desialylation is general, another BiTE molecule, PSMA BiTE, targeting prostate-specific membrane antigen (PSMA), was constructed. Again, a strong enhancement of cytotoxicity was observed when sialidase and PSMA BiTE were added simultaneously (Fig. 1d). To directly confirm that desialylation can enhance T-cell-dependent tumour-cell killing induced by BiTEs, we repeated the MCF-7 killing experiment using purified T cells. To our satisfaction, sialidase treatment indeed resulted in better BiTE-mediated target cell killing (Supplementary Fig. 1d). Moreover, the addition of B. infantis sialidase also substantially enhanced interferon-γ (IFNγ) secretion and T-cell activation (Fig. 1e,f).
Fig. 1 |. Removal of sialic acid enhances BiTE-induced T-cell cytotoxicity and activation.
a, Measurement of sialic acid levels on the surface of SK-BR-3 cells after treatment with B. infantis sialidase using the sialic acid-binding lectin SNA-FITC and MAL II-biotin and the galactose-binding lectin PNA-FITC. The MFI is shown in the figure. b, Killing of SK-BR-3 cells and MCF-7 cells induced by 4D5 BiTEs with or without sialidase treatment. c, Killing of SK-BR-3 cells induced by 4D5 BiTEs + hPBMCs with or without previous treatment with the 100 mM sialylation inhibitor P-3Fax-Neu5Ac. d, Killing of PSMA-positive PC3 cells induced by PSMA-targeting BiTEs with or without sialidase treatment. e, IFNγ release was measured as an indicator of BiTE-induced T-cell activation by incubating MCF-7 cells with 4D5 BiTEs + hPBMCs with or without the addition of sialidase. f, CD25 and CD69 expression levels were measured in T cells with or without the presence of 4D5 BiTEs and sialidase. The effector-to-target ratio used for all experiments in the figure is 5:1. Sialidase was added at a concentration of 1.5 mg ml−1. Mean values represent three independent experiments with s.e.m. as error bars. Unpaired Student’s t-test with Welch correction was used for statistical analysis.
Tumour-cell desialylation promotes stronger BiTE-mediated IS formation between T cells and tumour cells
Through their interaction with sialylated glycans aberrantly expressed on tumour cells, immune cell-associated Siglecs trigger signalling cascades to suppress immune cell activation and effector function20,21. Therefore, to probe the mechanism underlying the potentiation of BiTE-induced cytotoxicity by desialylation, we first investigated whether the sialoside–Siglec inhibitory pathway is involved. A previous study observed increased T-cell-dependent cytotoxicity of blinatumomab and catumaxomab, a trifunctional antibody targeting epithelial cell adhesion molecule, against sialic acid-deficient target tumour cells16. It was argued that the increased potency was due to the attenuation of the inhibitory signals mediated by the sialoside–Siglec-9 interaction. However, in agreement with previous reports, we found that CD3+ T cells from PBMCs of healthy donors expressed negligible levels of Siglec-7 and Siglec-9 compared with their CD3− counterparts, which consist mainly of B cells, natural killer (NK) cells, monocytes and dendritic cells (Fig. 2a,b). Nevertheless, we did observe a slight upregulation of both Siglec-7 and Siglec-9 following T-cell activation. Compared with the expression of these Siglecs on freshly isolated CD3− cells, the expression of Siglec-7 and Siglec-9 on activated CD3+ T cells was still minimal (Fig. 2a). To further test whether the Siglec-9 inhibitory pathway plays a role in T-cell-mediated target cell killing induced by BiTE, a Siglec-9-blocking antibody was added with 4D5 BiTE and the level of target cell killing was analysed. In contrast to sialidase addition, blocking of the Siglec-9 signals did not increase cytotoxicity, indicating a negligible role of Siglec-9 in BiTE-induced target cell killing in vitro (Fig. 2c). Although BiTE-induced killing is often considered to be independent of the CD28 co-stimulatory signal, several studies have reported therapeutic advantages when T-cell engager molecules are coupled with an additional CD28-recruiting moiety22–24. In addition, sialoglycans can block the interaction of CD28 with CD80, resulting in dampened co-stimulatory signalling14. To investigate whether the enhanced BiTE-induced killing seen with desialylation is influenced by CD28 co-stimulation, the CD28–CD80 interaction was blocked by the addition of a high-affinity ligand of CD80, recombinant human CTLA-4. However, even with the addition of high concentrations of CTLA-4, no changes in the enhanced cytolysis were detected in either the BiTE alone or BiTE plus sialidase groups (Fig. 2d).
Fig. 2 |. Desialylation promotes stronger BiTE-mediated IS formation rather than suppressing the inhibitory Siglec signalling.
a, Siglec-7 and Siglec-9 expression levels were measured on human T cells with or without BiTE-induced activation and with or without sialidase treatment. b, Siglec-7 and Siglec-9 expression levels were measured on T cells and CD3− cells in PBMCs from healthy human donors. c, SK-BR-3 cell killing induced by 4D5 BiTEs was measured with or without the addition of sialidase or anti-Siglec-9 antibody. d,e, SK-BR-3 cell killing induced by 4D5 BiTEs was measured in the presence or absence of sialidase with added recombinant CTLA-4 (d) and anti-CD2-blocking antibody (e). f, Staining of CD3ζ and F-actin to visualize the IS formed by T cells and tumour cells by confocal microscopy. Two groups, with and without sialidase treatment of the tumour cells, were imaged. Scale bar, 10 mm. g, CD3 accumulation at the IS was calculated by dividing the MFI at the IS by the MFI of the rest of the membrane. h, Relative IS contact area was calculated by dividing the area of the IS by the area of the rest of the T-cell membrane. All analyses were done using ImageJ. Mean values show three independent experiments with s.e.m. as error bars. For statistical analysis, unpaired Student’s t-test with Welch correction was applied.
The formation of BiTE-induced IS between target cells and T cells is the essential mode of action of BiTE molecules. We hypothesized that removal of cell-surface sialosides may lead to enhanced BiTE-induced IS formation between target tumour cells and T cells, thus promoting better tumour-cell killing. Since T-cell–target cell binding is the first step in triggering IS formation, we incubated HER2-positive SK-BR-3 cells or CD19-positive NALM-6 cells with PBMCs in the presence of 4D5 BiTE or CD19 BiTE, respectively, with or without sialidase treatment. As shown in Supplementary Fig. 2a,b, desialylation substantially increased BiTE-induced clustering between tumour cells and T cells. Similar trends were observed when PBMCs were replaced by purified T cells (Supplementary Fig. 2c).
Accumulation of the T-cell receptor–CD3 complex and F-actin at the synapse is a hallmark of a stable and functional cytolytic IS. To test whether desialylation could enhance IS formation, we imaged the IS formed between T cells with sialidase-treated and untreated SK-BR-3 cells by staining for F-actin and CD3ζ. The resulting immunofluorescence was imaged by confocal microscopy. As shown in Fig. 2f, we observed visually larger BiTE-induced IS formation between T cells and desialylated SK-BR-3 cells. To assess the stability of the formed IS, we calculated the relative CD3 fluorescence intensity at the IS and the relative area of the IS. The IS formed by sialidase-treated tumour cells and T cells showed substantially stronger CD3 accumulation and larger IS contact area compared with the IS formed by untreated tumour cells and T cells (Fig. 2g,h). The same trend was observed for BiTE-induced IS formation between SKOV-3 cells and T cells, with stronger IS formed following sialidase treatment (Supplementary Fig. 3).
The interaction between CD2 and CD58 is known to play a critical role in the formation of a productive IS25–27. We found that the inhibition of this interaction with an anti-CD2-blocking antibody partially reversed the cytotoxicity enhancement from the sialidase addition, further suggesting that the desialylation triggers stronger target cell killing by facilitating a tighter interaction between target tumour cells and T cells (Fig. 2e).
HER2-targeting BiTE–sialidase fusion protein selectively desialylates HER2-positive cells
Having confirmed that sialidase treatment potentiates the T-cell-dependent cytolysis of tumour cells induced by BiTE, we next sought to target sialidase specifically to the tumour-cell–T-cell interface by constructing a fusion protein. Confining sialidase activity to the target cells would potentiate tumour-cell killing while limiting non-specific desialylation of cells in the immune system. Importantly, sialyl-Lewis X, a sialylated tetrasaccharide, is essential for leukocyte tethering and rolling en route to sites of inflammation and tumour tissues. Non-specific desialylation would destroy this glycan epitope on leukocytes, thereby impeding their tumour homing and thus effective tumour control28–30. To this end, we constructed 4D5 BiTE–B. infantis sialidase fusion proteins in which sialidase was introduced onto either the N-terminus (sialidase–4D5 BiTE) or the C-terminus (4D5 BiTE–sialidase) of 4D5 BiTE, respectively (Fig. 3a). To test whether the fusion protein can remove sialic acids from the surface of tumour cells, SK-BR-3 (HER2+++) and SKOV-3 (HER2+++), a human ovarian adenocarcinoma cell line (Supplementary Fig. 4a; HER2+++ means high HER2 expression), were treated with sialidase–4D5 BiTE or 4D5 BiTE–sialidase, respectively. Both fusion proteins resulted in productive desialylation as evidenced by decreased SNA staining (Fig. 3b), which was accompanied by decreased MAL II staining and increased PNA staining (Fig. 3b). Importantly, a substantially higher desialylation efficiency was achieved with the fusion proteins than with B. infantis sialidase alone due to the induced proximity effect. At a concentration of 50 nM, when either sialidase fusion protein was used, the MAL II staining-associated mean fluorescence intensity (MFI) of target cells decreased to background levels with a concomitant increase in PNA staining. By contrast, the MAL II staining-associated MFI of sialidase-treated cells remained above 105 with little change observed in PNA staining (Fig. 3c). Interestingly, the C-terminal sialidase fusion (4D5 BiTE–sialidase) showed higher desialylation efficiency than the N-terminal fusion (sialidase–4D5 BiTE). Probably owing to the higher binding affinity of the anti-HER2 scFv than the anti-CD3 scFv, higher levels of desialylation were achieved on tumour cells than on T cells when 4D5 BiTE–sialidase was used (Supplementary Fig. 5). To determine whether the sialidase fusion proteins can selectively desialylate HER2-positive cells in the presence of HER2-negative cells, we mixed SKOV-3 (HER2+++) and MDA-MB-468 (HER2−) cells, followed by the addition of 4D5 BiTE–sialidase. At both 5 nM and 50 nM concentrations, 4D5 BiTE–sialidase selectively desialylated HER2-positive SKOV-3 cells while sparing the HER2-negative MDA-MB-468 cells, thus confirming its exclusive selectivity (Fig. 3d).
Fig. 3 |. Construction of 4D5 BiTE–sialidase fusion proteins for selective desialylation of HER2-positive cells.
a, Two fusion proteins are constructed by fusing sialidase to either the N- or C-terminus of the 4D5 BiTE. b, Measurement of sialic acid levels on the surface of SK-BR-3 and SKOV-3 cells after treatment with the fusion proteins and staining with the lectins SNA-FITC, MAL II-biotin and PNA-FITC. SNA staining was performed on both SK-BR-3 and SKOV-3 cells (top), while MAL II and PNA staining were performed on SK-BR-3 cells (bottom). The MFI is shown in the figure. c, Comparison of desialylation efficiency between 4D5 BiTE–sialidase, sialidase–4D5 BiTE and free sialidase at different concentrations as shown by MAL II and PNA staining. d, HER2-positive SKOV-3 cells and HER2-negative MDA-MB-468 cells were mixed and treated with 5 nM or 50 nM 4D5 BiTE–sialidase. Cell-surface sialylation was measured by FITC-SNA staining and flow cytometry analysis.
HER2-targeting BiTE–sialidase elicits greater in vitro T-cell-dependent cytotoxicity and T-cell effector function than the original BiTE
We then compared the T-cell-dependent cytotoxicity mediated by both fusion proteins with that of the original 4D5 BiTE. At the same concentration of 4 nM, both fusion proteins induced a higher level of T-cell-dependent cytolysis of SK-BR-3 and SKOV-3 cells than 4D5 BiTE (Fig. 4a,b). Specifically, in a dose–response assay, using SK-BR-3 and SKOV-3 cells as the target cells, tenfold and threefold lower half maximal effective concentration (EC50) values, respectively, were measured for 4D5 BiTE–sialidase than 4D5 BiTE (4D5 BiTE EC50 ≈ 200 pM) (Fig. 4c,d). Consistent with these findings, 4D5 BiTE–sialidase induced the highest levels of T-cell activation as measured by the expression of the T-cell activation markers CD25 and CD69 and the degranulation marker CD107a (Fig. 4e–g). The strongest release of cytokines, including interleukin (IL)-2, IFNγ and tumour necrosis factor (TNF), was also observed for 4D5 BiTE–sialidase-treated T cells. By contrast, sialidase–4D5 BiTE unexpectedly reduced the production of pro-inflammatory cytokines by T cells (Fig. 4h–j). A similar trend was observed for SKOV-3 cells, with 4D5 BiTE–sialidase inducing the strongest T-cell activation (Supplementary Fig. 6). Owing to its superior ability to induce T-cell-dependent tumour-cell killing, 4D5 BiTE–sialidase was selected for further studies.
Fig. 4 |. 4D5 BiTE–sialidase exhibits better activities than 4D5 BiTE for killing HER2-positive target cells and activating T cells.
a,b, Specific lysis of HER2-positive SK-BR-3 (a) and SKOV-3 (b) cells with 4 nM 4D5 BiTE or sialidase fusion proteins at an effector-to-target ratio of 5:1. c,d, Dose-dependent targeted killing with 4D5 BiTE or 4D5 BiTE–sialidase against SK-BR-3 (c) and SKOV-3 (d) cells. e–g, CD25 (e), CD69 (f) and CD107a (g) expression was measured in T-cell populations in the presence of SK-BR-3 cells and 4 nM 4D5 BiTE or the fusion protein. h–j, IFNγ (h), IL-2 (i) and TNF (j) release were measured for 4D5 BiTE or fusion protein induced T-cell activation in the presence of SK-BR-3 cells. k, The cytotoxicity enhancements induced by 4D5 BiTE–sialidase compared with 4D5 BiTE for cell lines with different HER2 expression levels. l, The specific lysis of MDA-MB-468 cells under 4 nM 4D5 BiTE or 4D5 BiTE–sialidase at an effector-to-target ratio of 5:1. m, Volcano plot of differentially expressed genes between T cells treated with 4D5 BiTE or 4D5 BiTE–sialidase when co-cultured with target MDA-MB-231 cells (genes with adjusted P value <0.01 are shown). Relevant differentiated genes are highlighted in red. FC, fold change. n, The heatmap of cytokine activities ranked by CytoSig. EC50 values were calculated from a sigmoidal dose–response curve model using Prism8. Statistical analysis was performed using unpaired Student’s t-test with Welch correction.
We first evaluated 4D5 BiTE–sialidase-mediated killing of cell lines with different levels of cell-surface HER2 expression: SK-BR-3 (HER2+++), SKOV-3 (HER2+++), MDA-MB-231 (HER2+), MDA-MB-435 (HER2+) and MDA-MB-468 (HER2−) (Supplementary Fig. 4a). At a concentration of 4 nM, compared with 4D5 BiTE, 4D5 BiTE–sialidase strongly augmented the killing of cells with low levels of HER2 (HER2+), for example, MDA-MB-231 and MDA-MB-435. Under these conditions, greater increases in killing were achieved than those measured for HER high (HER2+++) cells (SK-BR-3 and SKOV-3 cells) (94–203% versus 22–24%) (Fig. 4k and Supplementary Fig. 4c,d). These observations suggest that desialylation can increase the susceptibility of target cells that would normally be relatively resistant to BiTE-induced T-cell-dependent killing. Notably, 4D5 BiTE–sialidase did not trigger killing of HER2-negative MDA-MB-468 cells or mouse melanoma B16-F10 cells that express abundant sialoglycans, indicating exclusive specificity for HER2-positive cells (Fig. 4l and Supplementary Fig. 4b).
The above studies showed that the 4D5 BiTE–sialidase engaged T cells exhibit higher cytolytic activities and were better activated versus those engaged by 4D5 BiTE. Therefore, it was of interest to determine whether the improved effector T-cell function was due to transcriptional alterations induced by sialidase treatment. To systematically characterize such transcriptional changes, we performed whole transcriptome RNA sequencing (RNA-seq) analysis on either the 4D5 BiTE–sialidase- or the 4D5 BiTE-treated CD3+ T cells co-cultured with target MDA-MB-231 cells (Supplementary Fig. 7a). Volcano plot comparisons of messenger RNA between the 4D5 BiTE–sialidase- and the 4D5 BiTE-treated T cells showed that 2,101 transcripts were differentially expressed between these two groups (adjusted P < 0.01) (Fig. 4m). The most highly upregulated gene transcripts in 4D5 BiTE–sialidase-treated T cells encoded molecules critical for effector T-cell function, including cytolytic enzymes and cytokines (GZMB, LTA, LIF and IFNG), cytokine receptors (IL2RA) and transcriptional regulators (FOSB and BATF3). Notably, gene transcripts associated with memory phenotypes, such as LEF1 and TCF7, inhibitory receptors, such as CD96 and PDCD4, and molecules involved in regulatory T-cell generation, such as SMAD3, were largely downregulated. Gene set enrichment analysis (GSEA) highlighted multiple key pathways that were upregulated in the 4D5 BiTE–sialidase-treated T cells, including those associated with the cell cycle, transcriptional activity and cell metabolism. Considerably, the expression of transcripts involved in both oxidative phosphorylation and glycolysis was notably upregulated. By contrast, the enrichment of downregulated genes pertained to pathways associated with Wnt–β-catenin and transforming growth factor (TGF)-β signalling was observed (Supplementary Fig. 7b). Consistently, cytokine signalling analysis (CytoSig) revealed that pro-proliferative and inflammatory cytokines, IL-2, IL-12 and IL-15, had the most substantially increased activity in the 4D5 BiTE–sialidase-treated T cells, whereas the activity of the suppressive cytokine TGF-β3 was downregulated (Fig. 4n)31. Taken together, the 4D5 BiTE–sialidase-treated T cells were in a more effector-differentiated state with higher oxidative phosphorylation, glycolysis activities and effector functions compared with the 4D5 BiTE-treated T cells. In addition, transcriptome analysis also revealed a minimal mRNA expression level of all Siglec family proteins among the entire T-cell population, confirming the minor role of these proteins during the process of BiTE-induced target cell killing (Supplementary Fig. 7c).
HER2-targeting BiTE–sialidase promotes stronger IS formation compared with the original BiTE
To determine whether the 4D5 BiTE–sialidase fusion protein also enhances T-cell cytolytic efficacy and activation by triggering more productive IS formation, we examined synapse formation induced by 4D5 BiTE and 4D5 BiTE–sialidase between two HER2-positive cancer cell lines and the immortalized human T lymphocyte Jurkat cells via fluorescence three-dimensional (3D) confocal microscopy. In the IS induced by 4D5 BiTE–sialidase, a stronger accumulation of F-actin, CD3, HER2 and pZAP70 was clearly detected compared with that induced by 4D5 BiTE (Fig. 5a). Next, the total fluorescence intensities (TFIs) of these structural and signalling molecules and the HER2 antigen at the IS were quantified, which revealed that substantially higher TFIs of these molecules were enriched at the 4D5 BiTE–sialidase-induced synapse compared with that induced by 4D5 BiTE (Fig. 5b–e and Supplementary Fig. 8a–d). To compare the enrichment of key molecules in the IS induced by 4D5 BiTE and BiTE–sialidase, we quantified the fluorescence intensity across the entire cell–cell conjugates (Supplementary Fig. 8e). Points A and C indicate the ends of the cell–cell conjugates, and point B is where the IS is formed. The data showed that the 4D5–sialidase-induced IS had higher intensity as well as better accumulation of F-actin, CD3 and pZAP70 at the IS (point B) compared with the rest of the cells compared with the 4D5 BiTE-induced IS. These results strongly support that 4D5 BiTE–sialidase is capable of inducing a better quality IS than the unfused 4D5 BiTE.
Fig. 5 |. 4D5 BiTE–sialidase promotes stronger IS formation between SK-BR-3 cells and Jurkat cells.
a, Fluorescence confocal microscopy of SK-BR-3 cells and Jurkat cells treated with either 4D5 BiTE or 4D5 BiTE–sialidase. F-actin (blue), CD3 (red), HER2 (green) and pZAP70 (cyan) are shown in the figure for three sets of data for each condition. DIC stands for differential interference contrast. Scale bars, 10 mm. b–e, Quantification of F-actin (b), CD3 (c), HER2 (d) and pZAP70 (e) TFI at the synaptic area between SK-BR-3 cells and Jurkat cells. Unpaired Student’s t-test with Welch correction was used for statistical analysis.
BiTE–sialidase fusion proteins specific for CD19 and PSMA trigger enhanced in vitro cytotoxicity and T-cell activation
To evaluate whether the BiTE–sialidase fusion format could be applied to improve the efficacy of BiTE molecules targeting other tumour-associated antigens, we designed and constructed two additional BiTE–sialidase fusion molecules. The first was based on the FDA-approved drug blinatumomab that targets CD19, a cell-surface marker on B cells and B-cell malignancies. The second was derived from BiTE against PSMA, a target for prostate cancer treatment. As shown in Supplementary Fig. 9a, compared with blinatumomab (CD19 BiTE), the sialidase fusion counterpart induced much stronger cytotoxicity towards CD19-positive Raji cells with a fivefold lower EC50 (0.80 pM versus 4.26 pM). Likewise, much stronger killing of NALM-6, another CD19-positive cell line, was achieved by CD19 BiTE–sialidase (Supplementary Fig. 9a). At the same concentration of 5 pM, CD19 BiTE–sialidase induced much higher T-cell activation and degranulation than blinatumomab (Supplementary Fig. 9b–e). Consistent with greater T-cell activation, CD19 BiTE–sialidase also triggered stronger cytokine release (Supplementary Fig. 9f–h). As what we observed for CD19 BiTE–sialidase, PSMA BiTE–sialidase also induced better T-cell-dependent killing of PC3 cells that were engineered to express high levels of PSMA and stronger T-cell activation compared with PSMA BiTE (Supplementary Fig. 10a,b).
BiTE–sialidase enables better tumour control than BiTE in xenograft models in immune-deficient mice
Having demonstrated the superiority of BiTE–sialidase fusion proteins over the original BiTE molecules in terms of inducing T-cell-dependent cytolysis of tumour cells in vitro, we then sought to determine whether this enhanced efficacy could also be achieved in vivo. We chose a human tumour mouse xenograft model using the NOD-Prkdcem26Cd52IL2rgem26Cd22/NjuCrl coisogenic (NCG) immunodeficient mouse to compare the antitumour immunity induced by 4D5 BiTE–sialidase and 4D5 BiTE constructs32. On day 0, NCG mice were injected subcutaneously (s.c.) with 2.5 million luciferase-expressing SK-BR-3 (SK-BR-3-luc) cells, followed by intraperitoneal administration of 5 million hPBMCs. On day 7, these NCG mice were divided into three groups and then received an intravenous infusion of phosphate-buffered saline (PBS), 4D5 BiTE or 4D5 BiTE–sialidase, respectively (Fig. 6a). After 5 h, blood was collected from each mouse to measure the serum IFNγ levels. The 4D5 BiTE–sialidase group was found to harbour the highest level of serum IFNγ, while the 4D5 BiTE-treated group had little increase in IFNγ levels compared with the PBS control group (Fig. 6b). The BiTE administration was continued twice weekly until day 41, and a second dose of 2 million hPBMCs per mouse was administered on day 16. During this treatment course, tumour growth was monitored by longitudinal non-invasive bioluminescence imaging (BLI). As shown in Fig. 6c,d, the administration of 4D5 BiTE–sialidase considerably delayed tumour-cell growth in vivo compared with the 4D5 BiTE treatment and PBS control. Remarkably, tumours in two mice receiving the 4D5 BiTE–sialidase treatment were completely eradicated by the end of the treatment regimen (Fig. 6e). We next assessed the in vivo efficacy of the CD19 BiTE–sialidase fusion protein using an orthotopic xenograft mouse model of leukaemia. In this model, CD19+ NALM-6 cells (0.8 million) and hPBMCs (6 million) were injected intravenously (i.v.) into NCG mice on day 0 (Fig. 6f). The recipient mice were divided into four groups on day 3 and received intravenous infusion of PBS, 4D5 BiTE–sialidase, CD19 BiTE or CD19 BiTE–sialidase, respectively. Substantially slower tumour progression was observed in the CD19 BiTE–sialidase-treated group compared with the CD19 BiTE-treated group, demonstrating superior in vivo antitumour activity of the sialidase fusion protein (Fig. 6g,h). Notably, no apparent differences were detected between the PBS control group and the group receiving HER2-targeting 4D5 BiTE–sialidase, indicating that antitumour effects induced by the fusion protein are dependent on the target engagement on tumour cells (Fig. 6h).
Fig. 6 |. BiTE–sialidase exhibits better tumour control than BiTE in vivo.
a, Experimental timeline and treatment protocol for a HER2-positive SK-BR-3 breast cancer xenograft in NCG mice (n = 5). b, Serum IFNγ release was measured 5 h after the first drug treatment. c, Bioluminescence was measured twice a week to visualize changes in tumour volume. On day 41, one mouse in the PBS control group died. d,e, Bioluminescence was measured and calculated for each mouse as an indication of tumour burden. Tumour progression was followed by plotting the change in the group average (d) and individual (e) values over time. f, Experimental timeline and treatment protocol for a xenograft NALM-6 model of acute lymphoblastic leukaemia. g, Bioluminescence measured on days 3 and 7 is shown for different groups to compare tumour burden. h, Tumour burden was measured and calculated by following the bioluminescence signals (n = 5). One-way analysis of variance and Student’s t-test were used to analyse the differences between groups.
A BiTE–sialidase fusion protein shows therapeutic advantages over the parental BiTE in a syngeneic mouse model of melanoma
To further evaluate the efficacy of BiTE–sialidase fusion proteins in an immunocompetent syngeneic mouse model, we constructed a murine CD3-engaging BiTE and the corresponding BiTE–sialidase from the scFv fragments derived from the anti-murine CD3ε clone 17A2 and the anti-human epidermal growth factor receptor (EGFR) antibody cetuximab. A mouse melanoma cell line, B16-EGFR5 (B16-E5), expressing a chimeric mouse EGFR with six amino acid mutations to allow binding of cetuximab was chosen as the target cell. The fusion protein induced desialylation of B16-E5 cells in vitro as confirmed by SNA staining (Fig. 7a). To compare the antitumour activities of EGFR BiTE and EGFR BiTE–sialidase in vivo, we inoculated C57BL/6J mice with B16-E5 tumour cells (s.c.), followed by intratumoural administration of EGFR BiTE or EGFR BiTE–sialidase. (Owing to the low yields of both EGFR BiTE and EGFR BiTE–sialidase proteins, we chose the intratumoural injection to compare their in vivo efficacy because of the lower dose required to achieve effective drug concentrations in tumours.) While both groups conferred therapeutic benefits over the PBS control group, the EGFR BiTE–sialidase treatment significantly delayed tumour growth compared with the EGFR BiTE counterpart and also offered notable survival benefits to recipient mice (Fig. 7b,c). To profile the key effector cell type mediating the tumour control of EGFR BiTE–sialidase treatment, we depleted the CD8+ or CD4+ T-cell population in mice inoculated with B16-E5 tumour cells and treated different groups with the fusion protein (Supplementary Fig. 11a). Depletion of CD8+ T cells completely abolished the antitumour benefit of the EGFR BiTE–sialidase treatment, whereas depletion of CD4+ T cells had minimal effects (Supplementary Fig. 11b). Next, we investigated whether the BiTE–sialidase fusion protein could induce changes in the immune cell composition of the tumour microenvironment. A single high dose of EGFR BiTE or EGFR BiTE–sialidase was injected intratumourally on day 11 post-tumour inoculation. Tumours and tumour-draining lymph nodes were collected 3 days after the treatment (Fig. 7d), at which time point, the fusion protein-treated group had smaller tumour sizes compared with the BiTE-treated group (Fig. 7d). We found that in tumour-draining lymph nodes, both the EGFR BiTE- and the EGFR BiTE–sialidase-treated groups had substantially higher numbers of lymphocytes compared with the PBS control group with the BiTE–sialidase-treated group having the highest CD8+ T-cell counts (Fig. 7e,f). When analysing tumour-infiltrating immune cells, compared with the EGFR BiTE-treated groups, the BiTE–sialidase-treated group had substantially higher frequencies of CD8+ T cells and NK cells (CD45.2+CD3−NK1.1+) and lower frequency of myeloid cells (CD45.2+CD11b+NK1.1−) (Fig. 7g–k). However, no apparent differences in CD4+ T cells and dendritic cells (CD45.2+CD11c+) were observed. We then analysed CD8+ T cells in different groups and found that CD8+ T cells in the EGFR BiTE–sialidase-treated group were skewed to a more effector-like phenotype (Supplementary Fig. 11c,d). Together, these results demonstrate that the BiTE–sialidase fusion protein facilitates the transformation of a myeloid-rich, T-cell-poor tumour microenvironment that is immunosuppressive into a more immunopermissive one populated by NK and CD8+ T cells, which, in turn, resulted in substantially improved tumour control.
Fig. 7 |. EGFR BiTE–sialidase shows improved tumour control by altering the composition of tumour-infiltrating immune cells in a syngeneic mouse model of melanoma.
a, Measurement of sialic acid levels on the surface of B16-E5 cells after treatment of EGFR BiTE–sialidase with sialic acid-binding lectin SNA. The MFI is shown in the figure. b,c, Tumour growth curve (b) and survival percentage (c) of the B16-E5 mouse model under intratumoural treatment with PBS, EGFR BiTE and EGFR BiTE–sialidase. B16-E5 cells (0.6 million) were inoculated into C57BL/6J mice (s.c.) and 0.5 μg BiTE or 0.93 μg BiTE–sialidase was administered intratumourally on days 8, 12 and 14 (n = 5 for each group). d, Profiling of tumour-infiltrating cells in the B16-E5 mouse model. Left: B16-E5 cells (0.6 million) were inoculated into C57BL/6J mice and 1.5 μg BiTE and 2.8 μg BiTE–sialidase were administered intratumourally on day 11 before killing on day 14. Right: Tumour weight on day 14. e,f, Total cell counts of CD45.2+ (e) and CD8+ (f) cells in tumour lymph nodes isolated on day 14. LN, lymph node. g–k, Percentage of CD8+ T cells (g), NK cells (h), CD4+ T cells (i), CD11c-positive cells (j) and CD11b-positive NK1.1-negative cells (k) in the tumours of different treatment groups (n = 5). Unpaired Student’s t-test with Welch correction was used for statistical analysis. Kaplan–Meier with log-rank test and Cox regression for survival analysis.
Discussion
The concept of cancer progression facilitated by hypersialylation was introduced more than five decades ago, but the idea of pursuing desialylation as a therapeutic strategy for cancer treatment has met with mixed results since the first attempts in the 1970s33–35. Recently, the enthusiasm for this idea has been reinvigorated36,37. In fact, selective removal of sialylated Siglec ligands in the tumour microenvironment using an antibody–sialidase conjugate enhanced antitumour immunity and suppressed tumour progression in vivo in several mouse tumour models37,38. Mechanistically, desialylation facilitated the conversion of tumour-associated macrophages with immunosuppressive phenotypes to their antitumour counterparts.
In the current study, we demonstrate that targeted desialylation also benefits the BiTE-based therapy, which so far has had only limited success in the treatment of solid tumours. The BiTE–sialidase fusion proteins developed herein are able to trigger superior T-cell-dependent cytotoxicity against target cancer cells both in vitro and in vivo in both solid tumour and haematologic cancer models. Notably, the observed cytotoxicity enhancement resulting from desialylation appears to be independent of the inhibitory Siglec signalling pathways. Instead, desialylation elicits stronger IS formation between the engaged T cells and target cells. Indeed, recently published studies have revealed that bulky glycans on the surface of tumour cells result in suboptimal synapse formation induced by both CAR-T cells and BiTEs39. As a result of improved IS formation, T cells engaged by BiTE–sialidase fusion proteins exhibit increased activation, antigen sensitivity and effector function.
Although we have evidence that the desialylation-induced cytotoxicity enhancement in vitro within a short window of 24 h is independent of the Siglec signalling, recent studies have shown that tumour-infiltrating T cells can express high levels of Siglec-9 (ref. 16). In addition, 48 h following T-cell receptor stimulation, T cells upregulate Siglec-5 to counteract activation signals40. Therefore, desialylation by BiTE–sialidase fusion proteins may further facilitate long-term tumour-cell killing by disrupting the inhibitory sialoglycan–Siglec interaction on T cells and other immune cells. Finally, the overexpression of sialosides on tumour cells is known to contribute to tumour metastasis41–43. There is a possibility that BiTE–sialidase could suppress metastasis by inducing targeted desialylation44. These hypotheses are currently being investigated in our laboratory.
So far, many efforts have been devoted to improving the efficacy of T-cell-engaging therapies. One strategy is to incorporate the CD28 co-stimulatory signal into the T-cell-engaging process by either adding a CD28-engaging molecule or constructing tri-specific molecules with both the CD3- and CD28-binding moieties22–24. Others have attempted to incorporate immune checkpoint inhibitors45. BiTE-secreting CAR-T cells and BiTE-encoding oncolytic viruses have also been developed in an effort to achieve better efficacy46,47. In contrast to these strategies, we demonstrated through the development of BiTE–sialidase fusion proteins that the efficacy of BiTEs can be substantially enhanced by targeted editing of cell-surface glycocalyx. As illustrated in this work, the BiTE–sialidase fusion method is applicable to BiTEs targeting various tumour-associated targets, thus opening a new door for improving multiple types of T-cell-engaging therapy. We anticipate that BiTE–sialidase fusion proteins will prove to be promising agents that can be combined with other anti-cancer modalities such as immune checkpoint inhibitors and adoptive cell transfer to achieve better tumour control.
Methods
Cell lines and cell culturing
SK-BR-3 cells, MCF-7 cells, PC3 cells, Raji cells, SKOV-3 cells, MDA-MB-435 cells, MDA-MB-231 cells, MDA-MB-468 cells, NALM-6 cells and NK92MI cells were obtained from ATCC and were cultured as suggested by ATCC. The B16-E5 cell line was a kind gift from Yangxin Fu’s lab. A lentivirus transduction system was used to establish PSMA-positive PC3 cells in which PSMA-positive population was selected by FACS sorting. Expi293F cells were purchased from Thermo Fisher Scientific and cultured according to the manufacturer’s protocol. For culturing of the isolated human PBMCs, AIM V Medium (Gibco 12055091) supplemented with 10% FBS was used. All cells were cultured in an incubator at 37 °C supplemented with 5% CO2.
General gene cloning procedures
The protein sequences of scFv targeting human CD3, CD19, HER2 and PSMA were obtained from publicly available patents and the protein sequences were reverse-translated and codon-optimized to DNA sequences. All scFv sequences were synthesized by IDT. The sequences of EGFR and murine CD3-binding scFv were kindly provided by Yangxin Fu’s lab. The sequence of B. infantis sialidase was provided by Peng George Wang’s lab. For molecular cloning, the sequence fragments were assembled using NEBuilder HiFi DNA Assembly (New England BioLabs, E2621). For BiTE molecules, two individual scFv sequences were connected by a GGGGS linker. For all BiTE and sialidase fusion proteins, the sialidase sequence was fused to the BiTE sequence through a 2x GGGGS linker.
Expression of BiTEs, B. infantis sialidase and BiTE–sialidase fusion proteins
All BiTE, sialidase and BiTE–sialidase fusion proteins were fused with a 6x His tag at the C-terminus for purification. For all BiTE and BiTE–sialidase fusion proteins, the expression was done in an Expi293F cell system (Thermo Fisher Scientific). The transfection and handling of the cells were done according to the manufacturer’s protocol. B. infantis sialidase was expressed in BL21 Escherichia coli. For purification, all proteins were purified using Ni-NTA (nickel-nitrilotriacetic acid) resin from QIAGEN. After the incubation of the Expi293 media supernatant with the Ni-NTA resin, the nickel-charged resin was washed with PBS and 20 mM imidazole. Proteins were eluted with 250 mM imidazole and were concentrated and buffer-exchanged to PBS before use. The concentration of all proteins was determined by a Qubit protein quantification assay (Thermo Fisher Scientific, Q33211).
Desialylation by B. infantis sialidase, 4D5 BiTE–sialidase fusion proteins and P-3Fax-Neu5Ac
For the removal of sialic acids by B. infantis sialidase or 4D5 BiTE–sialidase fusion protein, 0.5 million cells were suspended in 100 μl DMEM without the serum. Sialidase or the fusion protein (final concentration: 1.5 mg ml−1) was added in each sample and each sample was incubated at 37 °C for an hour. After the incubation, cells were washed twice with DPBS before they were used for killing experiments or staining. For the desialylation by the inhibitor P-3Fax-Neu5Ac (R&D Systems, 117405–58-0), SK-BR-3 cells were cultured in a T25 flask with the addition of 100 mM P-3Fax-Neu5Ac for 3 days.
Desialylation detection from SNA, MAL II and PNA staining
For the SNA staining, 0.5 million cells with or without desialylation were suspended in 100 μl HBSS buffer (Sigma-Aldrich, H6648) supplemented with 5 mM CaCl2 and MgCl2. SNA-fluorescein isothiocyanate (FITC) was added at 1:200 and DAPI was added at 1:2,000 to each sample and the mixture was incubated on ice for 30 min before washing twice with HBSS buffer. Samples were then analysed by FACS. Desialylation was analysed in DAPI-negative live cell populations using FlowJo. The same procedures were used for PNA and MAL II staining.
Human PBMC and T-cell isolation
Human PBMCs were collected from blood samples of multiple healthy donors. In brief, an equal amount of DPBS with 2 mM EDTA was used to dilute the blood samples. Then the mixture was carefully added to Ficoll (Ficoll Paque Plus, GE Healthcare, 17–1440-02) for gradient separation. After centrifugation at 650 × g for 30 min with minimal acceleration and deceleration setting, the middle layer was collected and washed twice with DPBS supplemented with 2 mM EDTA. Further T-cell isolation from human PBMCs was done with an EasySep human T-cell isolation kit (STEMCELL Technologies, 100–0695) according to the manufacturer’s protocol.
Cell cytotoxicity measurement by lactate dehydrogenase release
T-cell cytotoxicity induced by BiTEs and BiTE–sialidase fusion proteins was measured by lactate dehydrogenase release using a CytoTox 96 non-radioactive cytotoxicity assay (Promega, G1780). Tumour cells (10,000) and hPBMCs (50,000) per well in 100 ml media were exposed to different treatments and incubated in 96-well plates at 37 °C (unless different ratios were specified elsewhere). After 24 h of co-incubation, 50 ml of the media supernatant from each well was transferred to a new flat-bottom 96-well plate and lactate dehydrogenase release was measured using the supplier′s protocol. Specific killing was calculated as suggested in the supplier′s protocol with background subtraction and total lysis comparison. Human recombinant CTLA-4 (Thermo Fisher, A42594), Siglec-9-blocking antibody (clone K8, BioLegend) and CD2-blocking antibody (clone RM2–5, eBioscience) were added in separate assays to test the effects of them for BiTE-induced killing.
Cytokine release and T-cell-surface activation marker measurement
For T-cell cytokine release measurement, as with the cytotoxicity experiment, 10,000 tumour cells and 50,000 hPBMCs were co-incubated per well in 96-well plates with different treatments in 100 ml media at 37 °C for 24 h. Then 20 ml of the supernatant from each well was diluted in 100 ml DPBS and used for IFNγ, IL-2 and TNF measurement. The ELISA measurement was done by ELISA MAX Sets. IFNγ, IL-2 and TNF kits (BioLegend) and the experiments were done according to the manufacturer’s protocol. The exact concentration was calculated from a standard curve. For the cell-surface activation marker measurement, 80,000 tumour cells and 400,000 hPBMCs were co-incubated per well in 12-well plates with different treatments in 1 ml media at 37 °C for 24 h. Following incubation, cells from each well were resuspended and stained with anti-CD3-PE, anti-CD69-FITC, anti-CD25-APC or anti-CD107a-Pacific blue (all from BioLegend and were added at 1:200) for 30 min at 4 °C. Cells were then washed twice with FACS buffer (PBS with 2.5% BSA) before being analysed using flow cytometry. Data analysis and MFI calculation were done by FlowJo. For the transcriptome analysis, 1.2 million hPBMCs and 0.1 million MDA-MB-231 cells were incubated together under the treatment of either 4 nM 4D5 BiTE or 4 nM 4D5 BiTE–sialidase (three replicas for each condition). After 48 h of incubation, the mixture was stained with DAPI and CD3 to sort out the T-cell population. mRNA of the T cell from each population was extracted by the Arcturus PicoPure RNA isolation kit (Thermo Fisher). The mRNA samples were sent out to Novogene for sequencing and initial analysis.
Flow cytometric analysis of Siglec-7 and Siglec-9 expression
Human PBMCs were collected from four healthy human donors. Freshly isolated human PBMCs (0.5 million) were suspended in 100 ml FACS buffer (PBS with 2.5% BSA) and each sample was stained with anti-CD3-PE. Each sample was also stained with either anti-Siglec-7-APC or anti-Siglec-9-APC (all from BioLegend and were added at 1:200). After incubation for 30 min at 4 °C, cells were washed twice with FACS buffer before being analysed using flow cytometry. Positive population percentage of both Siglec-7- and Siglec-9-stained samples was analysed by FlowJo. For T cells activated by BiTEs, 80,000 tumour cells and 400,000 hPBMCs were co-incubated per well in a 12-well plate with or without the BiTEs and sialidase treatment in 1 ml media at 37 °C for 24 h. Following incubation, cells were resuspended and stained as described earlier for Siglec-7 and Siglec-9 expression analysis.
Staining of human CD3ζ and actin for confocal imaging
In brief, 0.4 million tumour cells were treated with 4 nM 4D5 BiTE or 4 nM 4D5 BiTE with 15 mg ml−1 sialidase in 100 ml DMEM without the serum for 1 h at 37 °C. After the incubation, all the samples were washed twice using PBS before incubating with 0.4 million hPBMCs in 500 ml PBS for 30 min at 37 °C. Then all the cells were transferred in 1 ml PBS to the coverslips in 12-well plates and incubated at 37 °C for 30 min to let cells attach to the coverslip. One millilitre 4% paraformaldehyde was added to each well and incubated with shaking for 20 min at room temperature (RT) for cell fixing, and then each well was washed twice with ice-cold PBS. Washing took place at RT for 10 min with shaking. After fixation, 1 ml 0.1% PBS-Triton X-100 was added to each well for 10 min with shaking at RT to permeabilize the sample. PBST was used for washing three times, each time with shaking at RT for 5 min. Next, 1 ml 2.5% FBS-PBST was used to block each sample for 50 min with shaking at RT. Then anti-CD247 (CD3ζ) antibody (Sigma-Aldrich, 12–35-22–00) was diluted in FACS buffer at 1:200 and anti-actin antibody (Novus Biologicals, NBP267113) was diluted in 1:500. Each diluted antibody (500 ml) was added to samples and incubated for an hour at RT with shaking. PBST was used for washing three times before anti-rabbit IgG 488 (Invitrogen, 35553) and anti-mouse IgG 594 (Invitrogen, A-11005) secondary antibody was diluted and used for staining at RT for 30 min with shaking. Finally, samples were washed three times and each coverslip was transferred to a glass slide with mounting oil. Fingernail oil was used to seal the coverslip. Samples were analysed on a Zeiss LSM880 with a 63× oil lens (1.4 numerical aperture). The relative MFI of CD3ζ accumulation and relative contact area of IS were calculated by ImageJ.
Cluster formation analysis
For the cluster formation experiments between SK-BR-3 cells and T cells, 0.5 million SK-BR-3 and 1 million hPBMCs were stained with CellTracker Green CMFDA (Thermo Fisher) and PE anti-CD3 separately. After washing, they were incubated together under the treatment of the 50 nM 4D5 BiTE with or without the sialidase or 50 nM 4D5 BiTE–sialidase at 37 °C for 2 h before the sample was analysed by the FACS machine. The cluster experiment for the NALM-6 cells was of the same steps and settings, except that NALM-6 GL cells carry GFP expression that does not need CellTracker staining. The cluster experiment was also repeated with the purified T cells. All results were analysed by FlowJo.
IS formation and confocal imaging for BiTE–sialidase fusion protein
About 2 × 105 MCF-7 or SK-BR-3 cells were incubated with 10 nM 4D5 BiTE or 4D5 BiTE–sialidase in serum-free DMEM for 30 min at 37 °C. After incubation, the tumour cells were washed twice using PBS before incubating with 2 × 105 Jurkat cells. The tumour-cell lines and Jurkat cells were gently mixed and centrifuged at 300 rpm for 3 min and incubated for 15 min at 37 °C to allow conjugate formation. The mixed cells were then gently resuspended and allowed to settle on a poly-l-lysine-coated chamber slide for 30 min at 4 °C. The cells were fixed in freshly prepared 4% paraformaldehyde for 15 min and then permeabilized in 0.5% Triton X-100 and 10% normal donkey serum in PBS for 30 min at RT. Next, the mixed cells were stained with primary antibodies against CD3 (OKT3, BioLegend), HER2 (24D2, BioLegend), CD45 (2D1, BioLegend), pZAP70 (Tyr319, Cell Signaling Technology) and pLck (3A5, Santa Cruz Biotechnology) in staining buffer containing 3% normal donkey serum and 0.05% Triton X-100 and incubated in 4 °C overnight. The cells were then stained with fluorescently labelled secondary antibodies for 2 h at RT. F-actin was stained with Alexa Fluor 405-conjugated phalloidin (A30104, Thermo Fisher). The samples were imaged on an Olympus FV3000 confocal microscope using a 60× (1.35 numerical aperture) oil objective. 3D reconstruction and quantification of fluorescence intensity at the IS were analysed using Imaris 10.0 analysis software (Bitplane AG). Each assay was repeated at least twice.
RNA-seq analysis
Quality of raw sequencing reads was verified using FastQC (FastQC: a quality control tool for high-throughput sequence data (online); available online at http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Reads were aligned to Homo sapiens genome GRCh38 and genic reads quantified using STAR version 2.7.0f48. Normalization, differential expression analysis and principal component analysis were performed using R package DESeq2 v1.35.0. Heatmaps were constructed using R package ComplexHeatmap v2.12.0. R version 4.2.1 was used. Cytokine target expression analysis was performed using the Python implementation of CytoSig31. GSEA was performed using GSEA49.
Evaluation of the antitumour efficacy of BiTE–sialidase in an immunodeficient human tumour-cell line xenograft mouse model
All animal experiments were approved by the TSRI Animal Care and Use Committee. To compare the antitumour efficacy of 4D5 BiTE–sialidase and 4D5 BiTE–sialidase, 15 NCG (6-week-old male) mice (Charles River Laboratories) were injected with 5 × 106 human PBMCs (intraperitoneally (i.p.)) and 2.5 × 106 SK-BR-3 cells (s.c.) on day 0. On day 6, mice were imaged by BLI and divided into groups based on similar tumour burden within each group. On day 7, three groups were treated i.v. with PBS, 6 mg 4D5 BiTE and 10 mg 4D5 BiTE–sialidase, respectively. Blood was collected from each mouse 5 h following BiTE administration and the serum IFNγ level was measured using ELISA MAX (BioLegend). Drug treatment was continued twice weekly, and mice received a second dose of 2 × 106 human PBMCs (i.p.) on day 16. Tumour burden was imaged multiple times throughout the whole study process. For the BLI, 200 μl 15 g l−1 d-luciferin, potassium salt (GoldBio) was injected i.p. into each mouse and mice were imaged after 10 min using the IVIS imaging system (PerkinElmer). To compare the antitumour efficacy of CD19 BiTE–sialidase and CD19 BiTE–sialidase, 20 NCG (6-week-old male) mice (Charles River Laboratories) were injected with 6 × 106 human PBMCs (i.v.) and 0.8 × 106 NALM-6 cells (i.v.) on day 0. On day 3, all mice were imaged and divided into four groups. CD19 BiTE (1.5 μg), CD19 BiTE–sialidase (2.8 μg), 4D5 BiTE–sialidase and PBS were injected into different groups, respectively. Tumour size was measured by BLI as previously described until the death of the PBS control group.
Evaluation of the antitumour efficacy of BiTE–sialidase in a B16-E5 syngeneic mouse model
For the B16-E5 syngeneic mouse model, 15 C57BL/6J mice (6-week-old male) were injected with 0.6 × 106 B16-E5 cells s.c. on day 0. On day 8, tumour size was obtained by caliper measurement using the formula Volume = (Width2 × Length)/2 and mice were divided into different groups. Intratumoural injection of 0.5 μg EGFR BiTE, 0.93 μg EGFR BiTE–sialidase and PBS was given to mice in different groups on days 8, 12 and 14. Tumour size was recorded every 2 days until the mouse reached the endpoint of a tumour size of 1,000 mm3. For the tumour-infiltrating lymphocyte profiling, 15 C57BL/6J mice (6-week-old male) were also injected with 0.6 × 106 B16-E5 cells s.c. on day 0. On day 11, tumour size was measured and divided into three groups. EGFR BiTE (1.5 μg), EGFR BiTE–sialidase (2.8 μg) and PBS were injected intratumourally into tumours in different groups. On day 14, tumours were collected and tumour-infiltrating lymphocytes from each tumour of different groups were stained with multiple markers for different populations within the CD45.2 lymphocytes for the profiling. For the T-cell depletion experiment, 20 C57BL/6J mice (6-week-old male) were injected with 0.6 × 106 B16-E5 cells s.c. on day 0. On day 7, 150 μg CD4+ (clone GK1.5, BioXcell) or CD8+ T-cell (clone GK2.43, BioXcell) depleting antibody was injected i.p. into two individual groups of mice (n = 5). Next day, T-cell depletion was verified by analysing circulating blood T-cell population. On days 8, 11 and 13, three injections of 1 μg 4D5 BiTE–sialidase were given intratumourally in all groups. Tumour size was monitored as previously described.
Statistical analysis
Unless specified elsewhere, results are shown using GraphPad Prism version 8.0.0 with standard error of the mean (s.e.m.) as error bars; each dot represents a biological replicate. P values were calculated using the built-in data analysis function of Microsoft Excel or GraphPad Prism8.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary Material
Acknowledgements
This work was supported by the NIH (to P.W. R01AI154138, to J.R.T. R01AI143884 and to D.L. R01AI130197). J.Z. is a recipient of the Cancer Research Institute/Irvington postdoctoral fellowship. We thank N. Lerner and J. R. Cappiello for their critical comments on the paper and L. Liu and Y. Fu for providing the B16-E5 cell line.
Footnotes
Competing interests
P.W., Z.Y. and K.Q. were listed as inventors of Sialidase Fusion Molecules and Use, a US patent application no. 63/338,134 filed in May 2022. The other authors declare no competing interests.
Additional information
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41551-024-01202-w.
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. Gene-expression data obtained by RNA-seq are available from the NCBI Gene Expression Omnibus, via the accession number GSE245991. The raw and analysed datasets generated during the study are available for research purposes from the corresponding author on reasonable request. Source data are provided with this paper.
References
- 1.Goebeler M-E & Bargou RC T cell-engaging therapies—BiTEs and beyond. Nat. Rev. Clin. Oncol---. 17, 418–434 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Przepiorka D. et al. FDA approval: blinatumomab. Clin. Cancer Res. 21, 4035–4039 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Arvedson T. et al. Targeting solid tumors with bispecific T cell engager immune therapy. Annu. Rev. Cancer Biol. 6, 17–34 (2021). [Google Scholar]
- 4.Singh A, Dees S. & Grewal IS Overcoming the challenges associated with CD3+ T-cell redirection in cancer. Br. J. Cancer 124, 1037–1048 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munn DH & Bronte V. Immune suppressive mechanisms in the tumor microenvironment. Curr. Opin. Immunol. 39, 1–6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc. Natl Acad. Sci. USA 99, 10231–10233 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magalhães A, Duarte HO & Reis CA Aberrant glycosylation in cancer: a novel molecular mechanism controlling metastasis. Cancer Cell 31, 733–735 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Pinho SS & Reis CA Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer 15, 540–555 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Dube DH & Bertozzi CR Glycans in cancer and inflammation—potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 4, 477–488 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Pearce OM & Läubli H. Sialic acids in cancer biology and immunity. Glycobiology 26, 111–128 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues E. & Macauley MS Hypersialylation in cancer: modulation of inflammation and therapeutic opportunities. Cancers 10, 207 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murugesan G, Weigle B. & Crocker PR Siglec and anti-Siglec therapies. Curr. Opin. Chem. Biol. 62, 34–42 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Smith BA & Bertozzi CR The clinical impact of glycobiology: targeting selectins, Siglecs and mammalian glycans. Nat. Rev. Drug Discov. 20, 217–243 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edgar LJ et al. Sialic acid ligands of CD28 suppress costimulation of T cells. ACS Cent. Sci. 7, 1508–1515 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Büll C. et al. Sialic acid blockade suppresses tumor growth by enhancing T-cell-mediated tumor immunity. Cancer Res. 78, 3574–3588 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Stanczak MA et al. Self-associated molecular patterns mediate cancer immune evasion by engaging Siglecs on T cells. J. Clin. Invest. 128, 4912–4923 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter P. et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc. Natl Acad. Sci. USA 89, 4285–4289 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore PA et al. Application of dual affinity retargeting molecules to achieve optimal redirected T-cell killing of B-cell lymphoma. Blood J. Am. Soc. Hematol. 117, 4542–4551 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Sela DA et al. An infant-associated bacterial commensal utilizes breast milk sialyloligosaccharides. J. Biol. Chem. 286, 11909–11918 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macauley MS, Crocker PR & Paulson JC Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 14, 653–666 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hudak JE, Canham SM & Bertozzi CR Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat. Chem. Biol. 10, 69–75 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu L. et al. Trispecific antibodies enhance the therapeutic efficacy of tumor-directed T cells through T cell receptor co-stimulation. Nat. Cancer 1, 86–98 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Correnti CE et al. Simultaneous multiple interaction T-cell engaging (SMITE) bispecific antibodies overcome bispecific T-cell engager (BiTE) resistance via CD28 co-stimulation. Leukemia 32, 1239–1243 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skokos D. et al. A class of costimulatory CD28-bispecific antibodies that enhance the antitumor activity of CD3-bispecific antibodies. Sci. Transl. Med. 12, eaaw7888 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Leitner J, Herndler-Brandstetter D, Zlabinger GJ, Grubeck-Loebenstein B. & Steinberger P. CD58/CD2 is the primary costimulatory pathway in human CD28− CD8+ T cells. J. Immunol. 195, 477–487 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Demetriou P. et al. A dynamic CD2-rich compartment at the outer edge of the immunological synapse boosts and integrates signals. Nat. Immunol. 21, 1232–1243 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen Y. et al. Cancer cell-intrinsic resistance to BiTE therapy is mediated by loss of CD58 costimulation and modulation of the extrinsic apoptotic pathway. J. Immunother. Cancer 10, e004348 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ley K. & Kansas GS Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat. Rev. Immunol. 4, 325–336 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Sackstein R, Schatton T. & Barthel SR T-lymphocyte homing: an underappreciated yet critical hurdle for successful cancer immunotherapy. Lab. Invest. 97, 669–697 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sackstein R. Glycosyltransferase-programmed stereosubstitution (GPS) to create HCELL: engineering a roadmap for cell migration. Immunol. Rev. 230, 51–74 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang P. et al. Systematic investigation of cytokine signaling activity at the tissue and single-cell levels. Nat. Methods 18, 1181–1191 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eberle C, Rowe J, Fiore A, Mihalek R. & Festin S. 199 enhanced immune responses in human breast and colon cancer following checkpoint therapy in a CD34+ stem cell humanized NCG (HuCD34NCG) mouse model. J. Immunother. Cancer 8, A214 (2020). [Google Scholar]
- 33.Bekesi JG, Arneault GS, Walter L. & Holland JF Immunogenicity of leukemia L1210 cells after neuraminidase treatment. J. Natl Cancer Inst. 49, 107–118 (1972). [PubMed] [Google Scholar]
- 34.Bekesi JG, Roboz JP & Holland JF Therapeutic effectiveness of neuraminidase-treated tumor cells as an immunogen in man and experimental animals with leukemia. Ann. N. Y. Acad. Sci. 277, 313–331 (1976). [DOI] [PubMed] [Google Scholar]
- 35.Sedlacek H, Hagmayer G. & Seiler F. Tumor therapy of neoplastic diseases with tumor cells and neuraminidase. Cancer Immunol. Immunother. 23, 192–199 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao H, Woods EC, Vukojicic P. & Bertozzi CR Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc. Natl Acad. Sci. USA 113, 10304–10309 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gray MA et al. Targeted glycan degradation potentiates the anticancer immune response in vivo. Nat. Chem. Biol. 16, 1376–1384 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanczak MA et al. Targeting cancer glycosylation repolarizes tumor-associated macrophages allowing effective immune checkpoint blockade. Sci. Transl. Med. 14, eabj1270 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greco B. et al. Disrupting N-glycan expression on tumor cells boosts chimeric antigen receptor T cell efficacy against solid malignancies. Sci. Transl. Med. 14, eabg3072 (2022). [DOI] [PubMed] [Google Scholar]
- 40.Vuchkovska A. et al. Siglec-5 is an inhibitory immune checkpoint molecule for human T cells. Immunology 10.1111/imm.13470 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dobie C. & Skropeta D. Insights into the role of sialylation in cancer progression and metastasis. Br. J. Cancer 124, 76–90 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yogeeswaran G. & Salk P. Metastatic potential is positively correlated with cell surface sialylation of cultured murine tumor cell lines. Science 212, 1514–1516 (1981). [DOI] [PubMed] [Google Scholar]
- 43.Bresalier RS et al. Enhanced sialylation of mucin-associated carbohydrate structures in human colon cancer metastasis. Gastroenterology 110, 1354–1367 (1996). [DOI] [PubMed] [Google Scholar]
- 44.Bull C. et al. Targeted delivery of a sialic acid-blocking glycomimetic to cancer cells inhibits metastatic spread. ACS Nano 9, 733–745 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Herrmann M. et al. Bifunctional PD-1 × αCD3 × αCD33 fusion protein reverses adaptive immune escape in acute myeloid leukemia. Blood J. Am. Soc. Hematol. 132, 2484–2494 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Choi BD et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat. Biotechnol. 37, 1049–1058 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Heidbuechel JP & Engeland CE Oncolytic viruses encoding bispecific T cell engagers: a blueprint for emerging immunovirotherapies. J. Hematol. Oncol. 14, 1–24 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dobin A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Subramanian A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The main data supporting the results in this study are available within the paper and its Supplementary Information. Gene-expression data obtained by RNA-seq are available from the NCBI Gene Expression Omnibus, via the accession number GSE245991. The raw and analysed datasets generated during the study are available for research purposes from the corresponding author on reasonable request. Source data are provided with this paper.