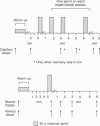
Abstract
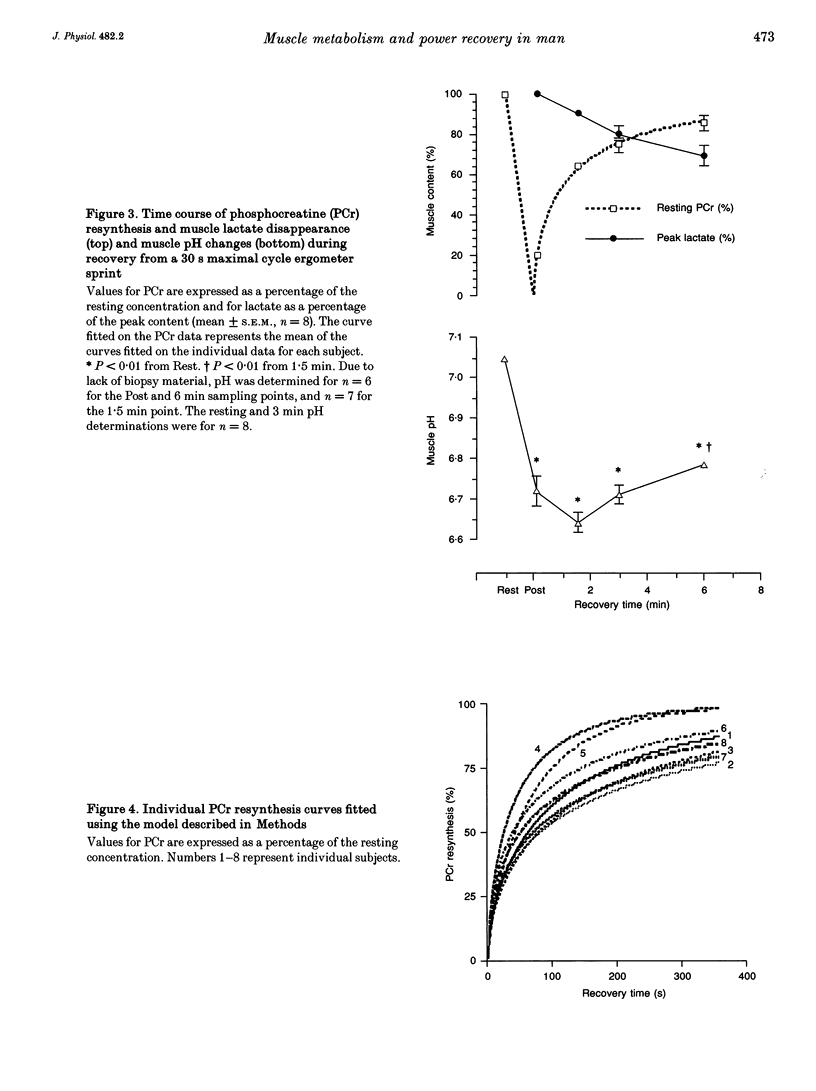
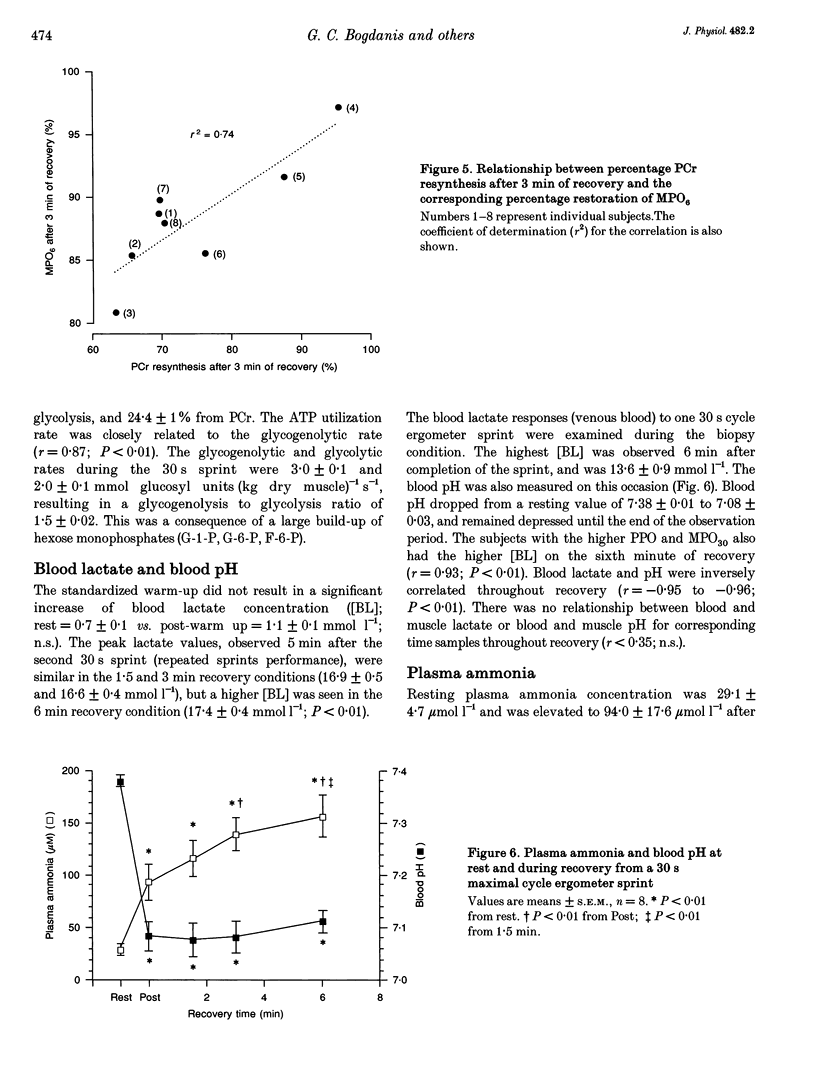
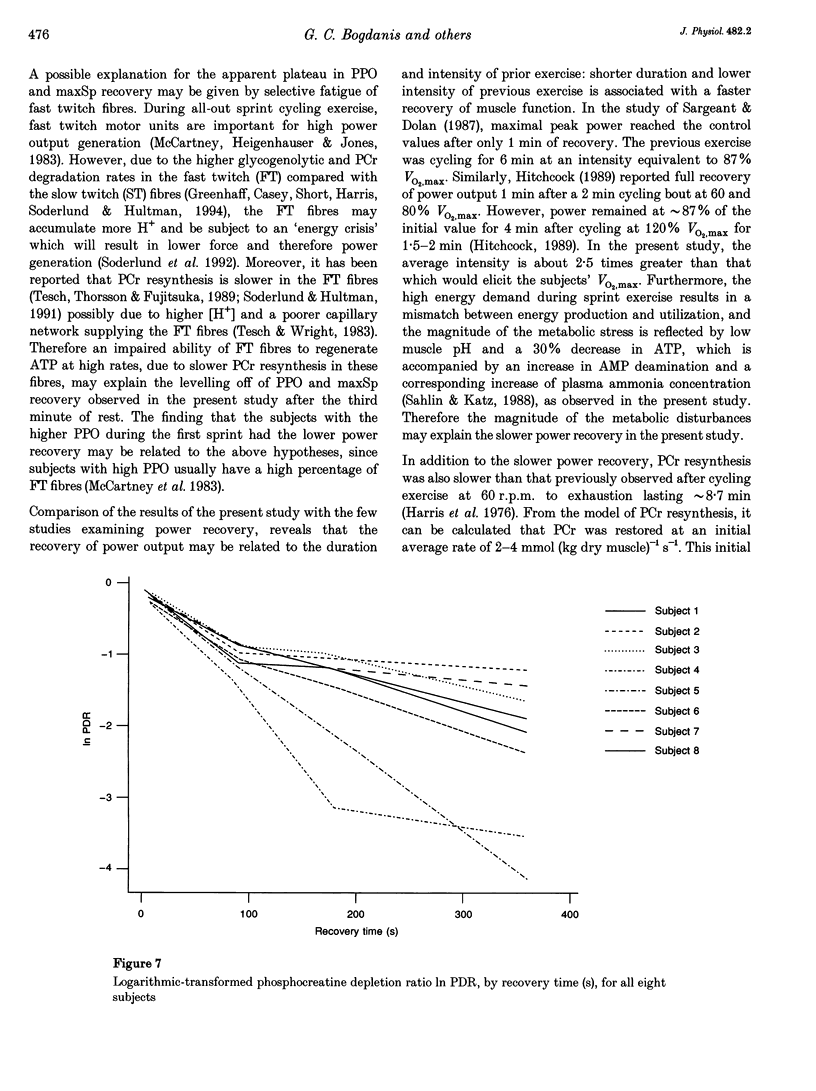
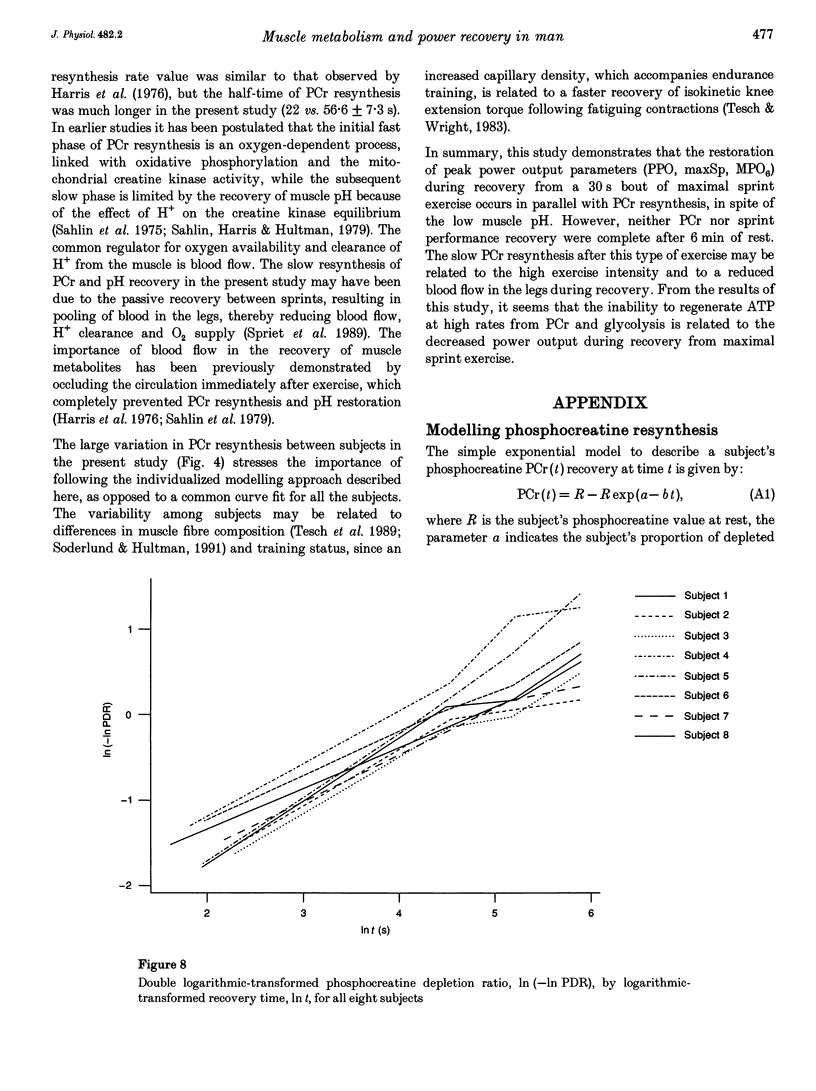
1. The recovery of power output and muscle metabolites was examined following maximal sprint cycling exercise. Fourteen male subjects performed two 30 s cycle ergometer sprints separated by 1.5, 3 and 6 min of recovery, on three separate occasions. On a fourth occasion eight of the subjects performed only one 30 s sprint and muscle biopsies were obtained during recovery. 2. At the end of the 30 s sprint phosphocreatine (PCr) and ATP contents were 19.7 +/- 1.2 and 70.5 +/- 6.5% of the resting values (rest), respectively, while muscle lactate was 119.0 +/- 4.6 mmol (kg dry wt)-1 and muscle pH was 6.72 +/- 0.06. During recovery, PCr increased rapidly to 65.0 +/- 2.8% of rest after 1.5 min, but reached only 85.5 +/- 3.5% of rest after 6 min of recovery. At the same time ATP and muscle pH remained low (19.5 +/- 0.9 mmol (kg dry wt)-1 and 6.79 +/- 0.02, respectively). Modelling of the individual PCr resynthesis using a power function curve gave an average half-time for PCr resynthesis of 56.6 +/- 7.3 s. 3. Recovery of peak power output (PPO), peak pedal speed (maxSp) and mean power during the initial 6 s (MPO6) of sprint 2 did not reach the control values after 6 min of rest, and occurred in parallel with the resynthesis of PCr, despite the low muscle pH. High correlations (r = 0.71-0.86; P < 0.05) were found between the percentage resynthesis of PCr and the percentage restoration of PPO, maxSp and MPO6 after 1.5 and 3 min of recovery. No relationship was observed between muscle pH recovery and power output restoration during sprint 2 (P > 0.05). 4. These data suggest that PCr resynthesis after 30 s of maximal sprint exercise is slower than previously observed after dynamic exercise of longer duration, and PCr resynthesis is important for the recovery of power during repeated bouts of sprint exercise.
Full text
PDF

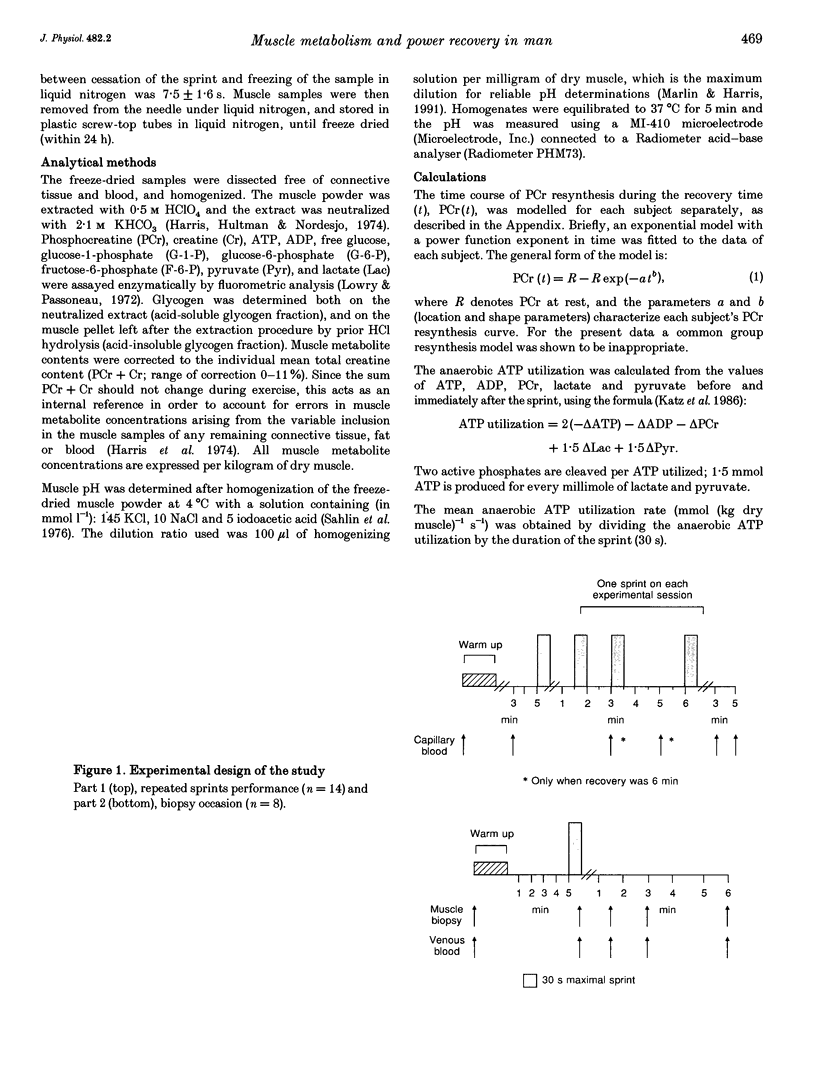

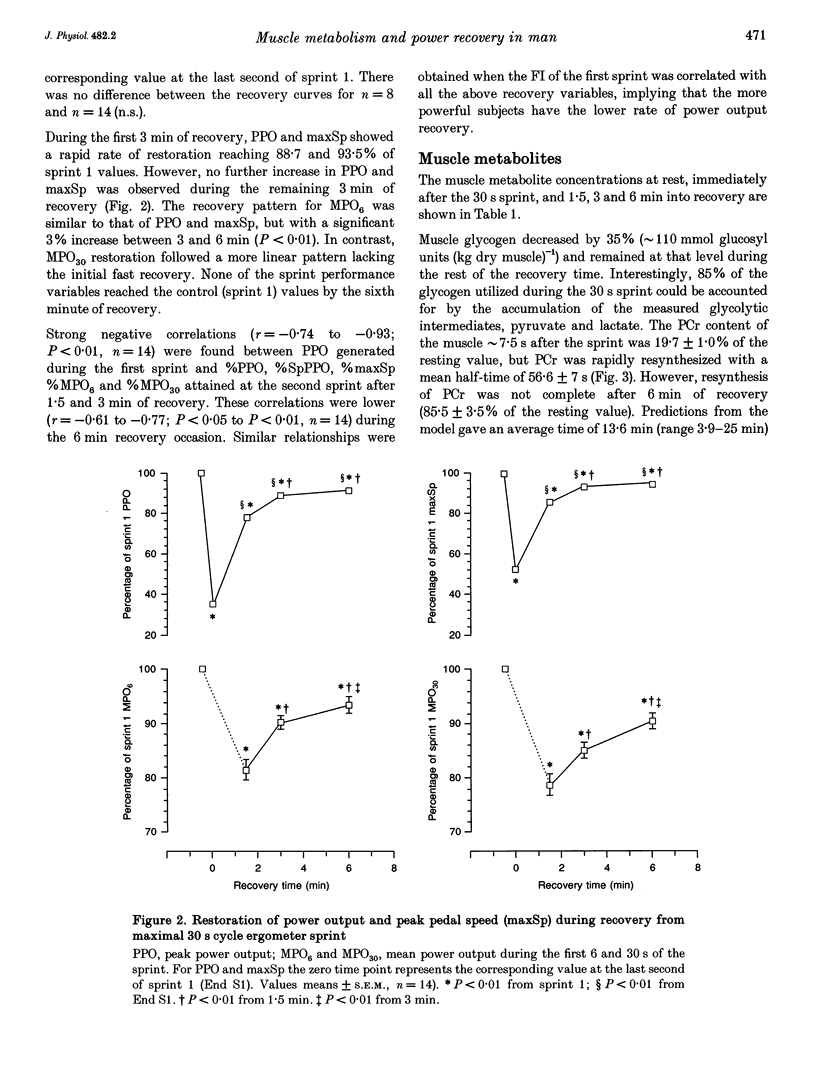
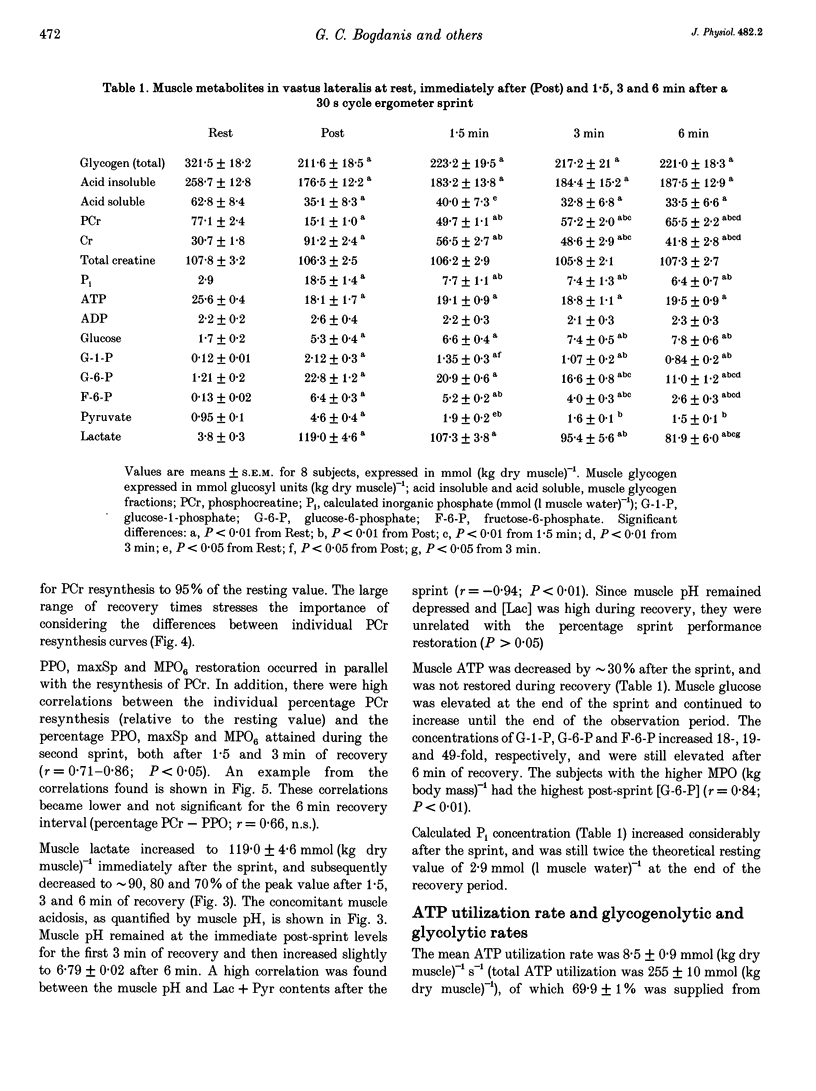



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker A. J., Carson P. J., Green A. T., Miller R. G., Weiner M. W. Influence of human muscle length on energy transduction studied by 31P-NMR. J Appl Physiol (1985) 1992 Jul;73(1):160–165. doi: 10.1152/jappl.1992.73.1.160. [DOI] [PubMed] [Google Scholar]
- Bergström M., Hultman E. Energy cost and fatigue during intermittent electrical stimulation of human skeletal muscle. J Appl Physiol (1985) 1988 Oct;65(4):1500–1505. doi: 10.1152/jappl.1988.65.4.1500. [DOI] [PubMed] [Google Scholar]
- Cady E. B., Elshove H., Jones D. A., Moll A. The metabolic causes of slow relaxation in fatigued human skeletal muscle. J Physiol. 1989 Nov;418:327–337. doi: 10.1113/jphysiol.1989.sp017843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasiotis D. The regulation of glycogen phosphorylase and glycogen breakdown in human skeletal muscle. Acta Physiol Scand Suppl. 1983;518:1–68. [PubMed] [Google Scholar]
- Cheetham M. E., Boobis L. H., Brooks S., Williams C. Human muscle metabolism during sprint running. J Appl Physiol (1985) 1986 Jul;61(1):54–60. doi: 10.1152/jappl.1986.61.1.54. [DOI] [PubMed] [Google Scholar]
- Dill D. B., Costill D. L. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974 Aug;37(2):247–248. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- Gaitanos G. C., Williams C., Boobis L. H., Brooks S. Human muscle metabolism during intermittent maximal exercise. J Appl Physiol (1985) 1993 Aug;75(2):712–719. doi: 10.1152/jappl.1993.75.2.712. [DOI] [PubMed] [Google Scholar]
- Greenhaff P. L., Casey A., Short A. H., Harris R., Soderlund K., Hultman E. Influence of oral creatine supplementation of muscle torque during repeated bouts of maximal voluntary exercise in man. Clin Sci (Lond) 1993 May;84(5):565–571. doi: 10.1042/cs0840565. [DOI] [PubMed] [Google Scholar]
- Greenhaff P. L., Nevill M. E., Soderlund K., Bodin K., Boobis L. H., Williams C., Hultman E. The metabolic responses of human type I and II muscle fibres during maximal treadmill sprinting. J Physiol. 1994 Jul 1;478(Pt 1):149–155. doi: 10.1113/jphysiol.1994.sp020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. C., Edwards R. H., Hultman E., Nordesjö L. O., Nylind B., Sahlin K. The time course of phosphorylcreatine resynthesis during recovery of the quadriceps muscle in man. Pflugers Arch. 1976 Dec 28;367(2):137–142. doi: 10.1007/BF00585149. [DOI] [PubMed] [Google Scholar]
- Harris R. C., Hultman E., Nordesjö L. O. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest. 1974 Apr;33(2):109–120. [PubMed] [Google Scholar]
- Harris R. C., Söderlund K., Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci (Lond) 1992 Sep;83(3):367–374. doi: 10.1042/cs0830367. [DOI] [PubMed] [Google Scholar]
- Hermansen L. Effect of metabolic changes on force generation in skeletal muscle during maximal exercise. Ciba Found Symp. 1981;82:75–88. doi: 10.1002/9780470715420.ch5. [DOI] [PubMed] [Google Scholar]
- Hitchcock H. C. Recovery of short-term power after dynamic exercise. J Appl Physiol (1985) 1989 Aug;67(2):677–681. doi: 10.1152/jappl.1989.67.2.677. [DOI] [PubMed] [Google Scholar]
- Jones N. L., McCartney N., Graham T., Spriet L. L., Kowalchuk J. M., Heigenhauser G. J., Sutton J. R. Muscle performance and metabolism in maximal isokinetic cycling at slow and fast speeds. J Appl Physiol (1985) 1985 Jul;59(1):132–136. doi: 10.1152/jappl.1985.59.1.132. [DOI] [PubMed] [Google Scholar]
- Katz A., Sahlin K., Henriksson J. Muscle ATP turnover rate during isometric contraction in humans. J Appl Physiol (1985) 1986 Jun;60(6):1839–1842. doi: 10.1152/jappl.1986.60.6.1839. [DOI] [PubMed] [Google Scholar]
- Lakomy H. K. Measurement of work and power output using friction-loaded cycle ergometers. Ergonomics. 1986 Apr;29(4):509–517. doi: 10.1080/00140138608968287. [DOI] [PubMed] [Google Scholar]
- Marlin D. J., Harris R. C. Titrimetric determination of muscle buffering capacity (beta mtitr) in biopsy samples. Equine Vet J. 1991 May;23(3):193–197. doi: 10.1111/j.2042-3306.1991.tb02753.x. [DOI] [PubMed] [Google Scholar]
- Maughan R. J. A simple, rapid method for the determination of glucose, lactate, pyruvate, alanine, 3-hydroxybutyrate and acetoacetate on a single 20-mul blood sample. Clin Chim Acta. 1982 Jul 1;122(2):231–240. doi: 10.1016/0009-8981(82)90282-0. [DOI] [PubMed] [Google Scholar]
- McCartney N., Heigenhauser G. J., Jones N. L. Power output and fatigue of human muscle in maximal cycling exercise. J Appl Physiol Respir Environ Exerc Physiol. 1983 Jul;55(1 Pt 1):218–224. doi: 10.1152/jappl.1983.55.1.218. [DOI] [PubMed] [Google Scholar]
- McCartney N., Spriet L. L., Heigenhauser G. J., Kowalchuk J. M., Sutton J. R., Jones N. L. Muscle power and metabolism in maximal intermittent exercise. J Appl Physiol (1985) 1986 Apr;60(4):1164–1169. doi: 10.1152/jappl.1986.60.4.1164. [DOI] [PubMed] [Google Scholar]
- Nevill A. M., Cooke C. B., Holder R. L., Ramsbottom R., Williams C. Modelling bivariate relationships when repeated measurements are recorded on more than one subject. Eur J Appl Physiol Occup Physiol. 1992;64(5):419–425. doi: 10.1007/BF00625060. [DOI] [PubMed] [Google Scholar]
- Nevill M. E., Boobis L. H., Brooks S., Williams C. Effect of training on muscle metabolism during treadmill sprinting. J Appl Physiol (1985) 1989 Dec;67(6):2376–2382. doi: 10.1152/jappl.1989.67.6.2376. [DOI] [PubMed] [Google Scholar]
- Sahlin K., Harris R. C., Hultman E. Creatine kinase equilibrium and lactate content compared with muscle pH in tissue samples obtained after isometric exercise. Biochem J. 1975 Nov;152(2):173–180. doi: 10.1042/bj1520173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin K., Harris R. C., Hultman E. Resynthesis of creatine phosphate in human muscle after exercise in relation to intramuscular pH and availability of oxygen. Scand J Clin Lab Invest. 1979 Oct;39(6):551–558. doi: 10.3109/00365517909108833. [DOI] [PubMed] [Google Scholar]
- Sahlin K., Harris R. C., Nylind B., Hultman E. Lactate content and pH in muscle obtained after dynamic exercise. Pflugers Arch. 1976 Dec 28;367(2):143–149. doi: 10.1007/BF00585150. [DOI] [PubMed] [Google Scholar]
- Sahlin K., Ren J. M. Relationship of contraction capacity to metabolic changes during recovery from a fatiguing contraction. J Appl Physiol (1985) 1989 Aug;67(2):648–654. doi: 10.1152/jappl.1989.67.2.648. [DOI] [PubMed] [Google Scholar]
- Sargeant A. J., Dolan P. Effect of prior exercise on maximal short-term power output in humans. J Appl Physiol (1985) 1987 Oct;63(4):1475–1480. doi: 10.1152/jappl.1987.63.4.1475. [DOI] [PubMed] [Google Scholar]
- Sjöholm H., Sahlin K., Edström L., Hultman E. Quantitative estimation of anaerobic and oxidative energy metabolism and contraction characteristics in intact human skeletal muscle in response to electrical stimulation. Clin Physiol. 1983 Jun;3(3):227–239. doi: 10.1111/j.1475-097x.1983.tb00706.x. [DOI] [PubMed] [Google Scholar]
- Spriet L. L., Lindinger M. I., McKelvie R. S., Heigenhauser G. J., Jones N. L. Muscle glycogenolysis and H+ concentration during maximal intermittent cycling. J Appl Physiol (1985) 1989 Jan;66(1):8–13. doi: 10.1152/jappl.1989.66.1.8. [DOI] [PubMed] [Google Scholar]
- Spriet L. L., Söderlund K., Bergström M., Hultman E. Skeletal muscle glycogenolysis, glycolysis, and pH during electrical stimulation in men. J Appl Physiol (1985) 1987 Feb;62(2):616–621. doi: 10.1152/jappl.1987.62.2.616. [DOI] [PubMed] [Google Scholar]
- Söderlund K., Greenhaff P. L., Hultman E. Energy metabolism in type I and type II human muscle fibres during short term electrical stimulation at different frequencies. Acta Physiol Scand. 1992 Jan;144(1):15–22. doi: 10.1111/j.1748-1716.1992.tb09262.x. [DOI] [PubMed] [Google Scholar]
- Söderlund K., Hultman E. ATP and phosphocreatine changes in single human muscle fibers after intense electrical stimulation. Am J Physiol. 1991 Dec;261(6 Pt 1):E737–E741. doi: 10.1152/ajpendo.1991.261.6.E737. [DOI] [PubMed] [Google Scholar]
- Tesch P. A., Thorsson A., Fujitsuka N. Creatine phosphate in fiber types of skeletal muscle before and after exhaustive exercise. J Appl Physiol (1985) 1989 Apr;66(4):1756–1759. doi: 10.1152/jappl.1989.66.4.1756. [DOI] [PubMed] [Google Scholar]
- Tesch P. A., Wright J. E. Recovery from short term intense exercise: its relation to capillary supply and blood lactate concentration. Eur J Appl Physiol Occup Physiol. 1983;52(1):98–103. doi: 10.1007/BF00429033. [DOI] [PubMed] [Google Scholar]