Abstract
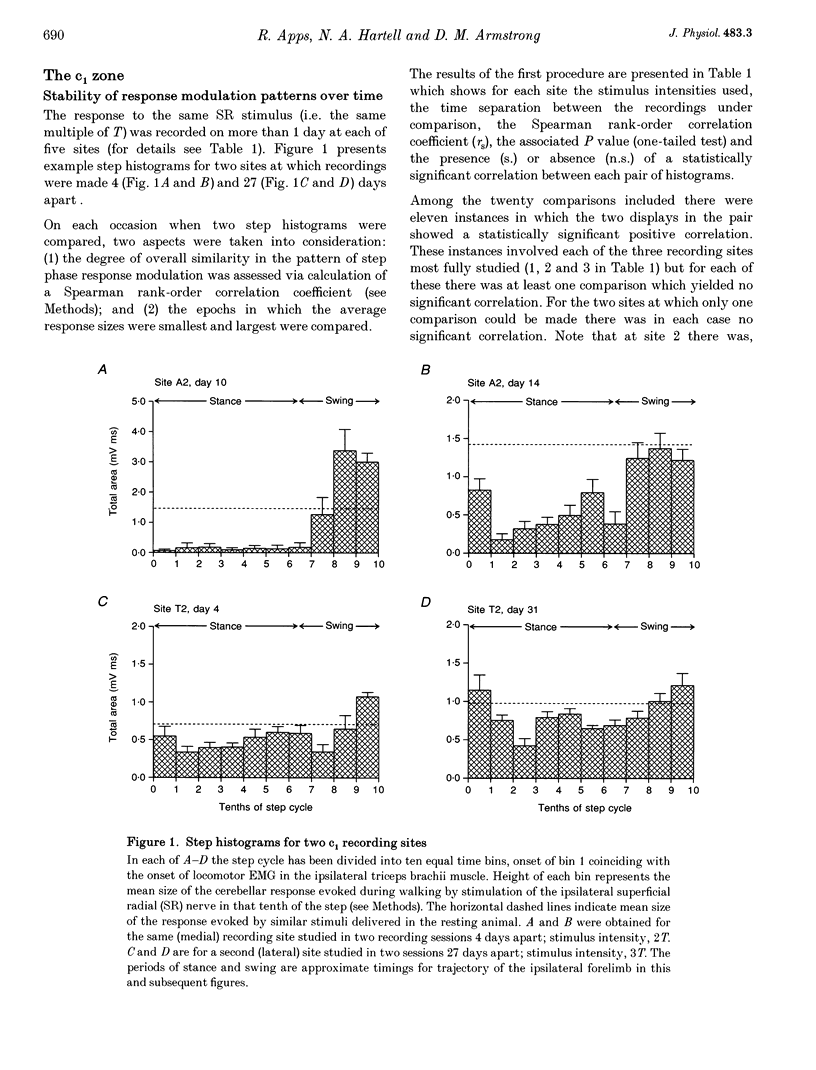
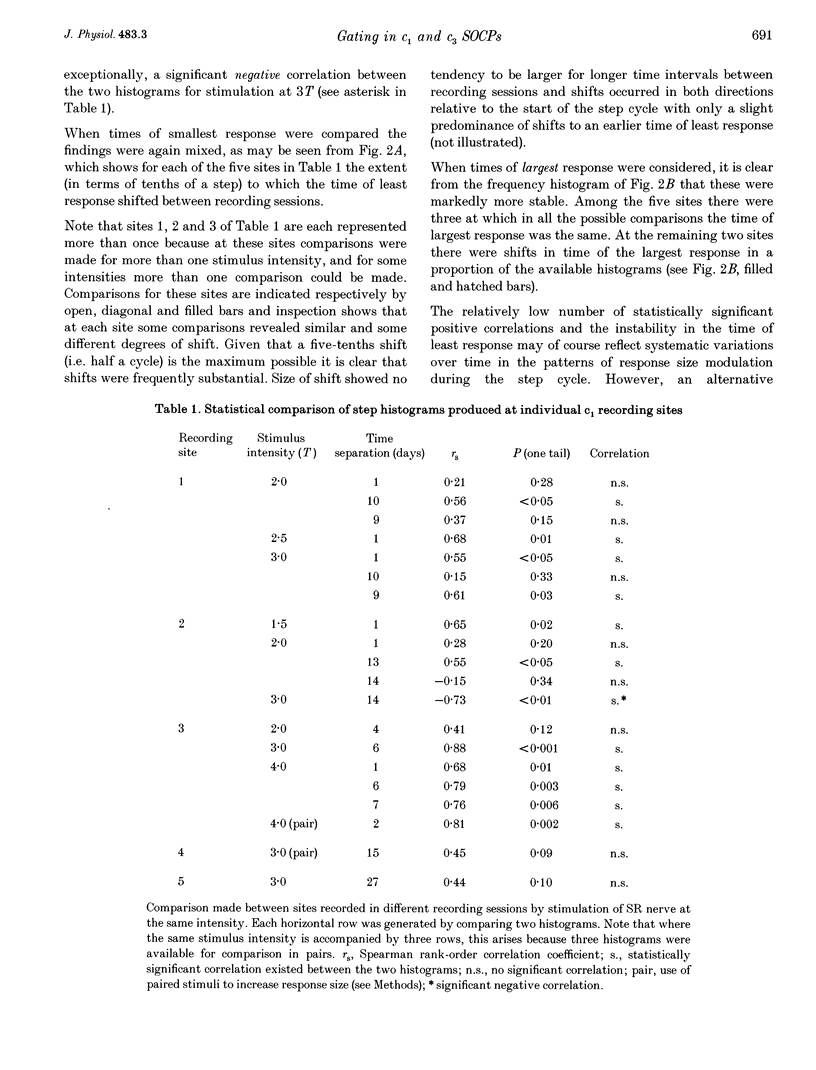
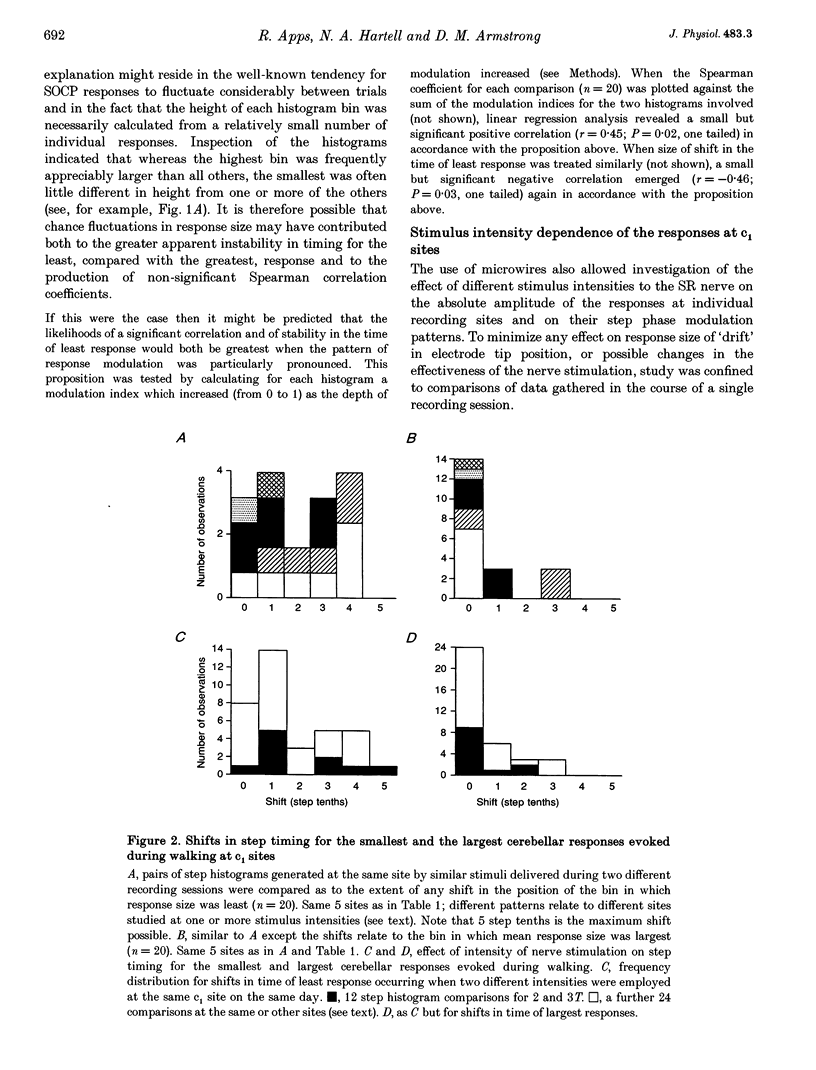
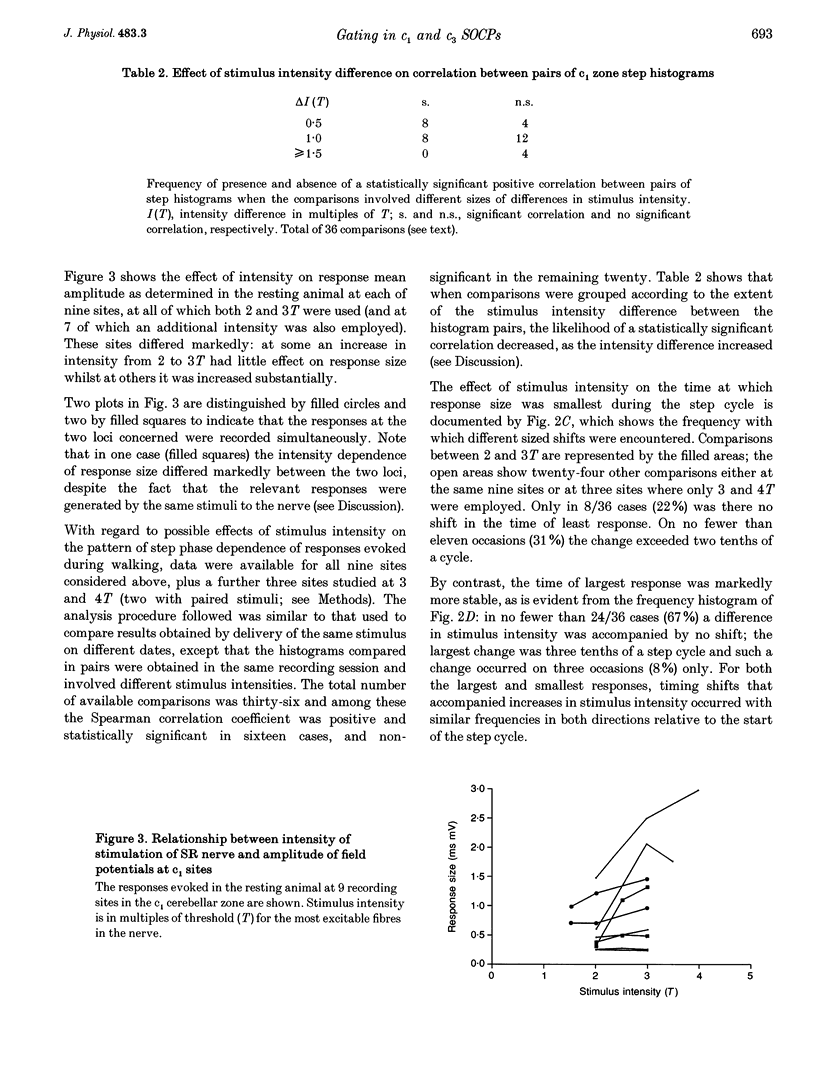
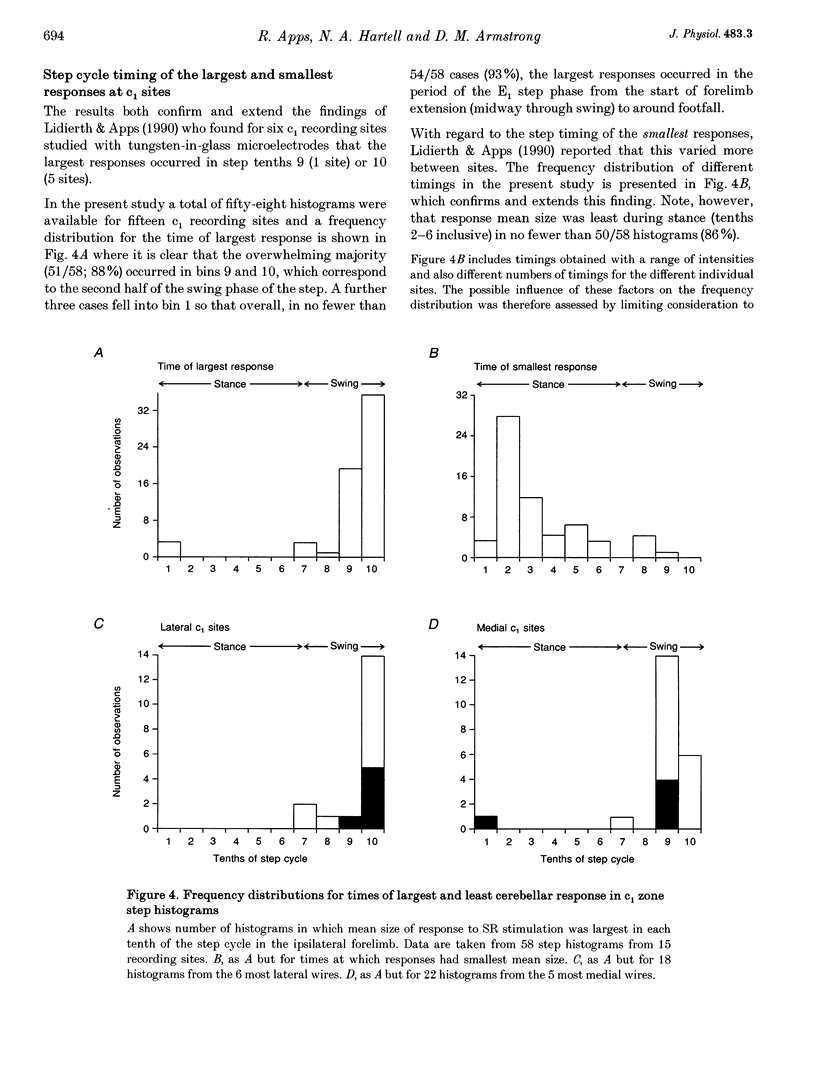
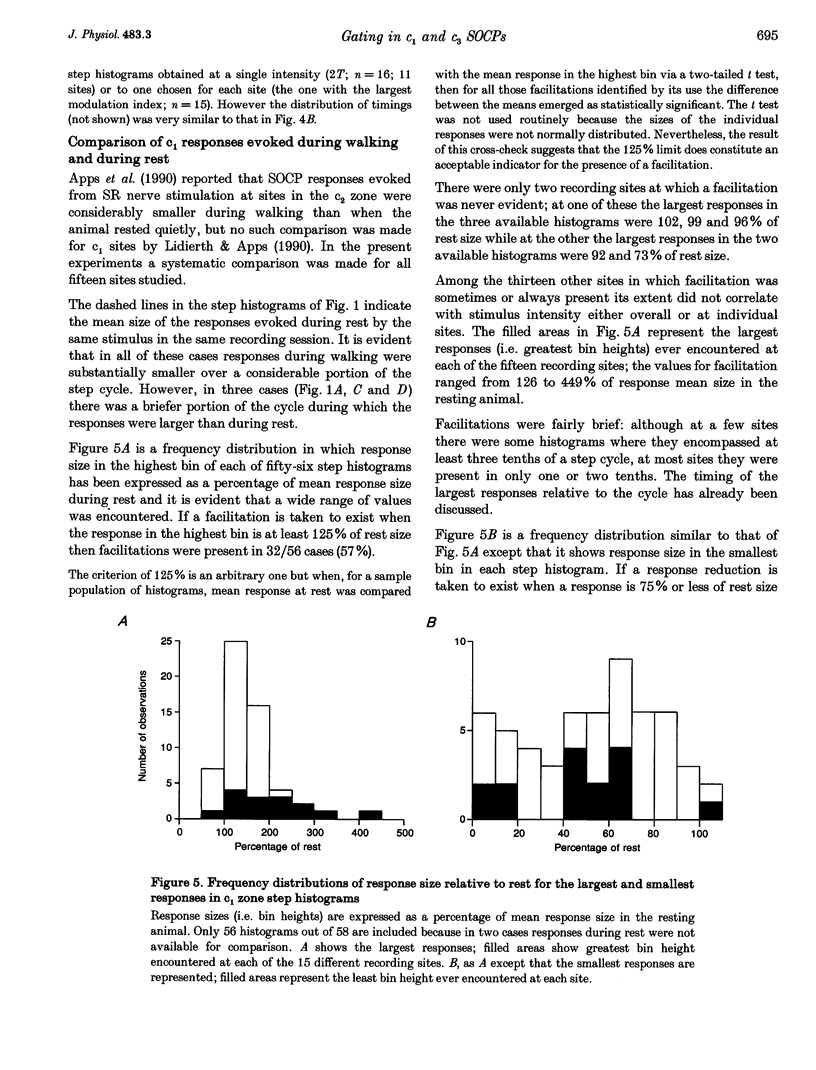
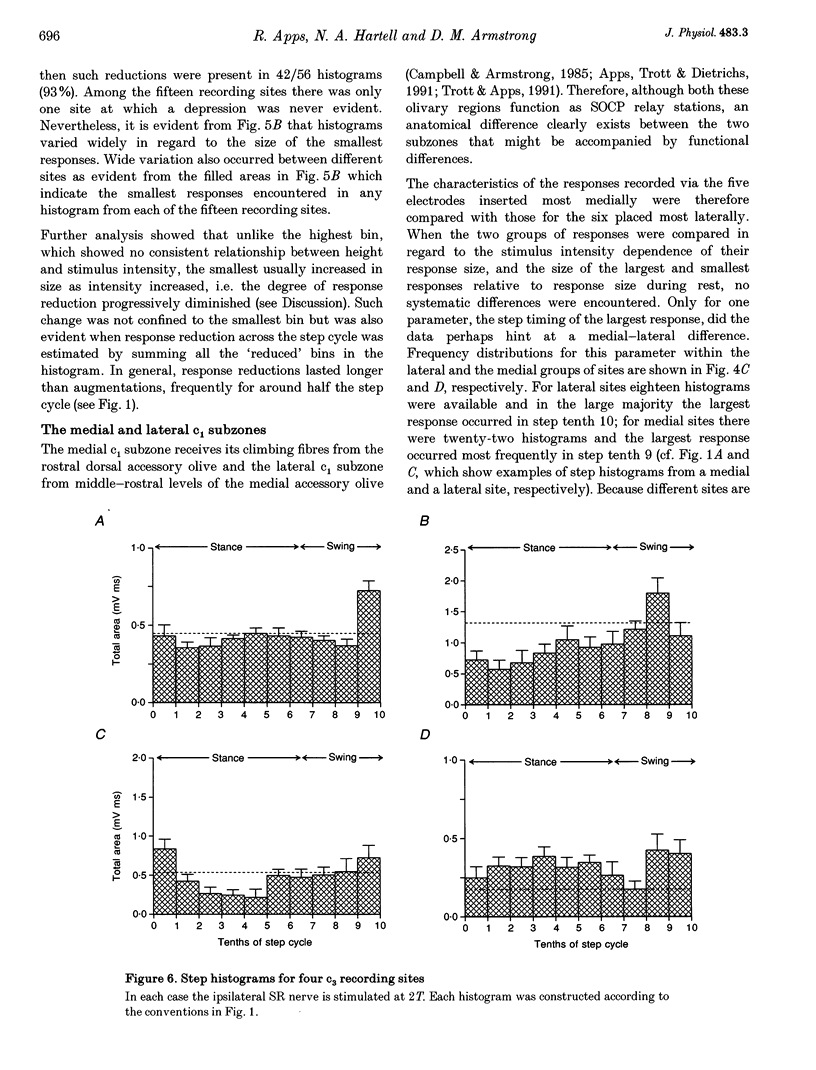
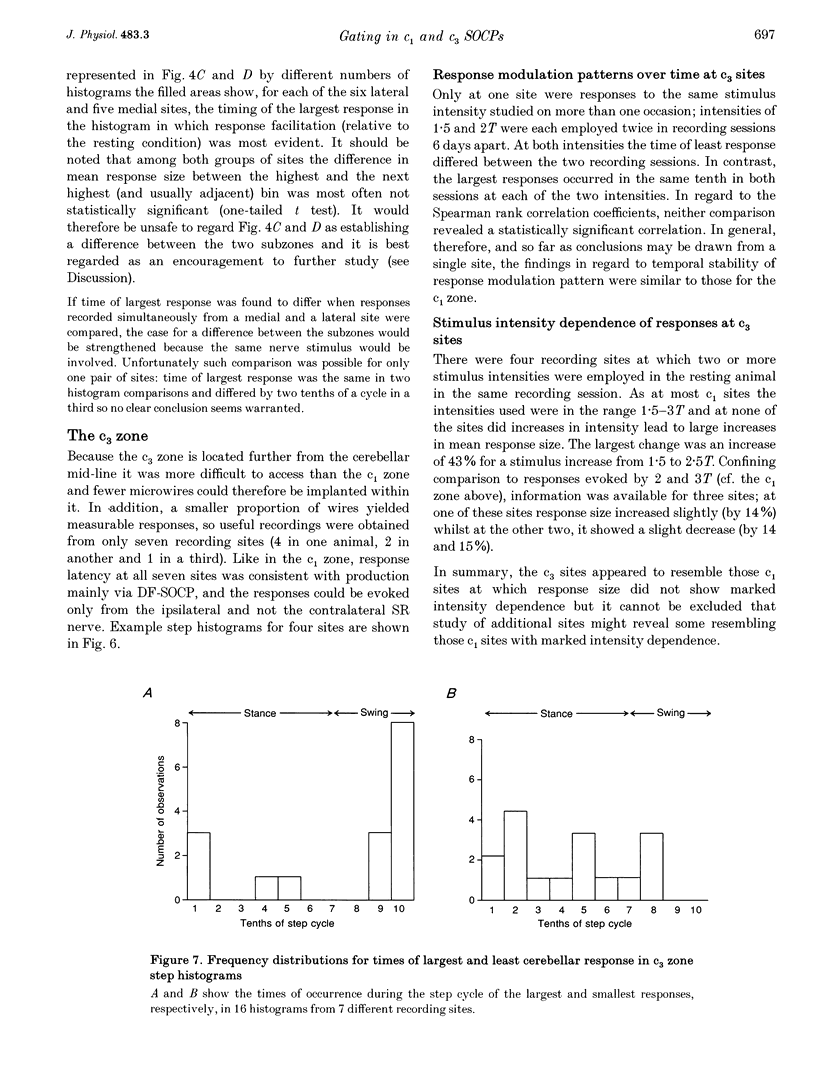
1. Chronically implanted microwires were used to record extracellular field potentials generated in the c1 and c3 zones in the cortex of lobules V and VI of the cerebellum by non-noxious stimuli delivered to the superficial radial nerve in the ipsilateral forelimb. Responses due to input via climbing fibre afferents were studied; their latency and other characteristics identified them as mediated mainly via the dorsal funiculus spino-olivocerebellar path (DF-SOCP). 2. Responses at individual sites were studied repeatedly with a range of stimulus intensities and during two different behaviours: quiet rest and steady walking on an exercise belt. For responses during walking, step histograms were constructed showing response mean size during different tenths of the step cycle in the ipsilateral forelimb, both in absolute terms and relative to mean size during rest. 3. Step histograms for the same site on different days or different stimulus intensities varied appreciably in form but in both cases the timing of the largest response was usually the same or shifted by only one step tenth. 4. In both zones the largest responses during walking occurred overwhelmingly during the E1 step phase when the limb is extended forwards and down to establish footfall. Least responses were much less uniform in timing but were mostly during stance, particularly its early (E2) part. 5. In many histograms the smallest responses were smaller in mean size than the responses during rest while the largest were larger. These changes were not paralleled by changes in nerve volley size, so presumably reflect step-related central changes in pathway excitability. Facilitations and depressions were differently affected by stimulus intensity and sometimes occurred independently, suggesting generation by separate mechanisms. 6. In both zones there were differences between recording sites which suggests that different DF-SOCP subcomponents innervate different parts of the zones. However, no systematic differences could be firmly established between the medial and lateral subzones of the c1 zone. 7. The results are discussed in relation to the hypothesis that the DF-SOCP constitutes the afferent limb of a transcerebellar mechanism involved in adapting the evolving step.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apps R., Lidierth M., Armstrong D. M. Locomotion-related variations in excitability of spino-olivocerebellar paths to cat cerebellar cortical c2 zone. J Physiol. 1990 May;424:487–512. doi: 10.1113/jphysiol.1990.sp018079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps R., Trott J. R., Dietrichs E. A study of branching in the projection from the inferior olive to the x and lateral c1 zones of the cat cerebellum using a combined electrophysiological and retrograde fluorescent double-labelling technique. Exp Brain Res. 1991;87(1):141–152. doi: 10.1007/BF00228515. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., Drew T. Discharges of pyramidal tract and other motor cortical neurones during locomotion in the cat. J Physiol. 1984 Jan;346:471–495. doi: 10.1113/jphysiol.1984.sp015036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Edgley S. A. Discharges of Purkinje cells in the paravermal part of the cerebellar anterior lobe during locomotion in the cat. J Physiol. 1984 Jul;352:403–424. doi: 10.1113/jphysiol.1984.sp015300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMBERS W. W., SPRAGUE J. M. Functional localization in the cerebellum. I. Organization in longitudinal cortico-nuclear zones and their contribution to the control of posture, both extrapyramidal and pyramidal. J Comp Neurol. 1955 Aug;103(1):105–129. doi: 10.1002/cne.901030107. [DOI] [PubMed] [Google Scholar]
- CHAMBERS W. W., SPRAGUE J. M. Functional localization in the cerebellum. II. Somatotopic organization in cortex and nuclei. AMA Arch Neurol Psychiatry. 1955 Dec;74(6):653–680. doi: 10.1001/archneurpsyc.1955.02330180071008. [DOI] [PubMed] [Google Scholar]
- Campbell N. C., Armstrong D. M. Origin in the medial accessory olive of climbing fibres to the x and lateral c1 zones of the cat cerebellum: a combined electrophysiological/WGA-HRP investigation. Exp Brain Res. 1985;58(3):520–531. doi: 10.1007/BF00235868. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966 Jan;182(2):268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S. A., Lidierth M. Step-related discharges of Purkinje cells in the paravermal cortex of the cerebellar anterior lobe in the cat. J Physiol. 1988 Jul;401:399–415. doi: 10.1113/jphysiol.1988.sp017169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot C. F., Garwicz M., Schouenborg J. Topography and nociceptive receptive fields of climbing fibres projecting to the cerebellar anterior lobe in the cat. J Physiol. 1991 Sep;441:257–274. doi: 10.1113/jphysiol.1991.sp018750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot C. F., Larson B. Branching of olivary axons to innervate pairs of sagittal zones in the cerebellar anterior lobe of the cat. Exp Brain Res. 1982;48(2):185–198. doi: 10.1007/BF00237214. [DOI] [PubMed] [Google Scholar]
- Ekerot C. F., Larson B. The dorsal spino-olivocerebellar system in the cat. I. Functional organization and termination in the anterior lobe. Exp Brain Res. 1979 Jul 2;36(2):201–217. doi: 10.1007/BF00238905. [DOI] [PubMed] [Google Scholar]
- Ekerot C. F., Larson B. The dorsal spino-olivocerebellar system in the cat. II. Somatotopical organization. Exp Brain Res. 1979 Jul 2;36(2):219–232. doi: 10.1007/BF00238906. [DOI] [PubMed] [Google Scholar]
- Garwicz M., Ekerot C. F. Topographical organization of the cerebellar cortical projection to nucleus interpositus anterior in the cat. J Physiol. 1994 Jan 15;474(2):245–260. doi: 10.1113/jphysiol.1994.sp020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellman R., Gibson A. R., Houk J. C. Inferior olivary neurons in the awake cat: detection of contact and passive body displacement. J Neurophysiol. 1985 Jul;54(1):40–60. doi: 10.1152/jn.1985.54.1.40. [DOI] [PubMed] [Google Scholar]
- Larson B., Miller S., Oscarsson O. Termination and functional organization of the dorsolateral spino-olivocerebellar path. J Physiol. 1969 Aug;203(3):611–640. doi: 10.1113/jphysiol.1969.sp008882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidierth M., Apps R. Gating in the spino-olivocerebellar pathways to the c1 zone of the cerebellar cortex during locomotion in the cat. J Physiol. 1990 Nov;430:453–469. doi: 10.1113/jphysiol.1990.sp018301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marple-Horvat D. E., Gilbey S. L. A method for automatic identification of periods of muscular activity from EMG recordings. J Neurosci Methods. 1992 May;42(3):163–167. doi: 10.1016/0165-0270(92)90095-u. [DOI] [PubMed] [Google Scholar]
- Palmer C. A microwire technique for recording single neurons in unrestrained animals. Brain Res Bull. 1978 May-Jun;3(3):285–289. doi: 10.1016/0361-9230(78)90129-6. [DOI] [PubMed] [Google Scholar]
- Shapovalov A. I. Neuronal organization and synaptic mechanisms of supraspinal motor control in vertebrates. Rev Physiol Biochem Pharmacol. 1975;72:1–54. doi: 10.1007/BFb0031545. [DOI] [PubMed] [Google Scholar]
- Trott J. R., Apps R. Lateral and medial sub-divisions within the olivocerebellar zones of the paravermal cortex in lobule Vb/c of the cat anterior lobe. Exp Brain Res. 1991;87(1):126–140. doi: 10.1007/BF00228514. [DOI] [PubMed] [Google Scholar]
- Trott J. R., Armstrong D. M. The cerebellar corticonuclear projection from lobule Vb/c of the cat anterior lobe: a combined electrophysiological and autoradiographic study. I. Projections from the intermediate region. Exp Brain Res. 1987;66(2):318–338. doi: 10.1007/BF00243308. [DOI] [PubMed] [Google Scholar]
- Tsukahara N., Toyama K., Kosaka K. Electrical activity of red nucleus neurones investigated with intracellular microelectrodes. Exp Brain Res. 1967;4(1):18–33. doi: 10.1007/BF00235214. [DOI] [PubMed] [Google Scholar]
- Udo M., Matsukawa K., Kamei H., Oda Y. Cerebellar control of locomotion: effects of cooling cerebellar intermediate cortex in high decerebrate and awake walking cats. J Neurophysiol. 1980 Jul;44(1):119–134. doi: 10.1152/jn.1980.44.1.119. [DOI] [PubMed] [Google Scholar]


