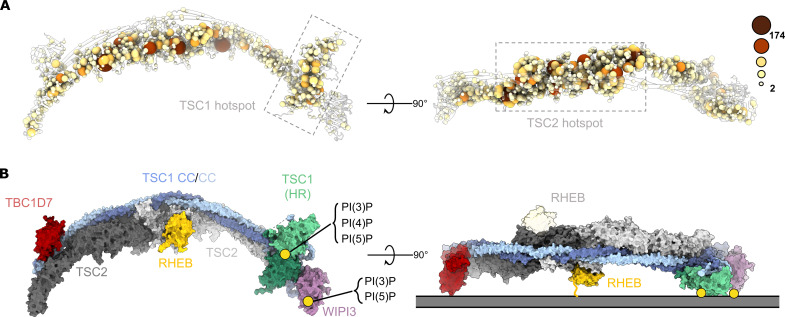
Fig. 4. TSC:WIPI3 disease–associated mutations and model of the TSC:WIPI3:RHEB lysosomal mTORC1 inhibitory complex.
(A) Cα atoms of all disease-associated missense mutations are rendered as spheres of size and color proportional to the frequency of observation (COSMIC, LOVD, and HGMD) (55–57). Disease-associated mutations cluster to the TSC1 HR PIP-binding dimer (left), as well as the TSC2 Rap-GAP central core (right). (B) Surface rendering of the complete human TSC with RHEB modelled according to AlphaFold (58). The binding sites of RHEB, TSC1:PI(3)P, and WIPI3:PI(3)P define a membrane binding plane that is consistent with a singly occupied RHEB-binding model of the TSC:WIPI3:RHEB lysosomal mTORC1 inhibitory complex.