Fig. 3 |. DUX4-mediated pathways and possible methods of therapeutic inhibition.
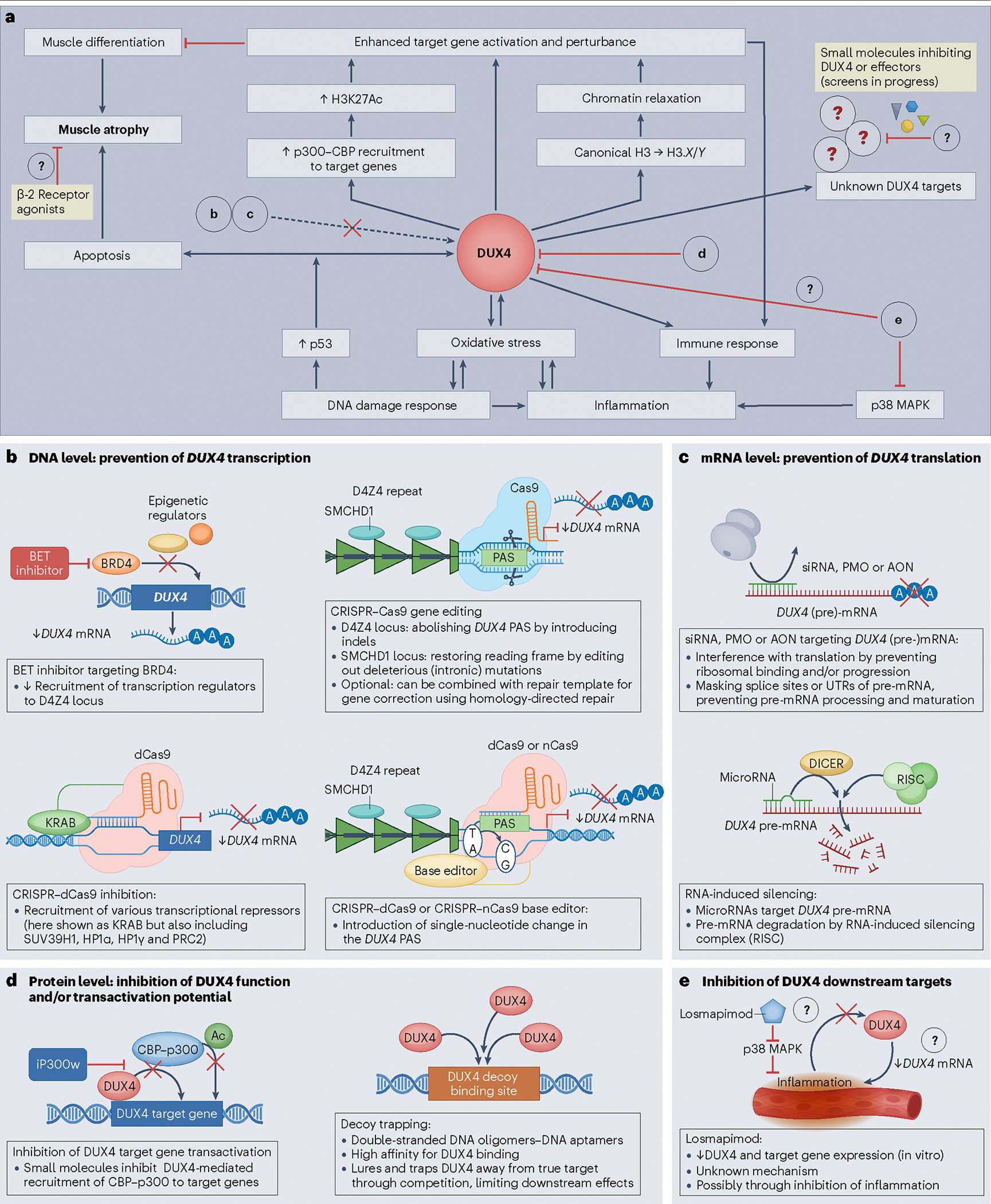
a, Pathways that are (partly) upregulated by and/or respond to DUX4 protein in skeletal muscle lead to transcriptional deregulation, inflammation and muscle atrophy. Possible interventions targeting either DUX4 function or DUX4 effectors are indicated with red inhibitory arrows. Bold letters refer to detailed figure panels showing the potential mechanisms that can attenuate the cytotoxic effects of DUX4. b, Top left. Bromodomain-containing protein 4 (BRD4) is an epigenetic regulator that activates DUX4 transcription by recruiting additional epigenetic regulators to the D4Z4 repeat. Bromodomain and extraterminal domain (BET) inhibitors block this BRD4-dependent recruitment and reduce DUX4 transcription. b, Bottom left. Clustered regularly interspaced short palindromic repeat (CRISPR)–dCas9 (‘dead’ caspase 9) inhibition recruits Krüppel-associated box (KRAB) zinc finger proteins and other transcriptional repressors to D4Z4, thereby reducing transcription of DUX4 and/or DUX4 target genes. b, right. CRISPR–Cas9 editing disrupts the polyadenylation signal (PAS), either through (top) inducing insertion–deletion mutations (indels) via double-stranded DNA breaks (traditional Cas9) or by (bottom) targeted conversion of a single DNA base pair using dCas9 or Cas9 nickase (nCas9) fused to a base editor protein. c, Phosphorodiamidate morpholino oligomers (PMOs), small interfering (si) RNAs or antisense oligonucleotides (AONs) bind to DUX4 transcripts and prevent maturation of pre-messenger RNA (mRNA) or ribosomal binding to mature mRNAs, thereby blocking translation. MicroRNAs that target DUX4 pre-mRNA can induce degradation of the RNA through the endoribonuclease DICER-activated RNA-induced silencing complex. d, At the protein level, DUX4 function can be blocked by the small molecule iP300w, which interferes with DUX4-dependent recruitment of the cyclic adenosine monophosphate response element binding protein (CREB)–p300 lysine acetyltransferase transcriptional activator complex, thereby inducing transcriptional deregulation. Alternatively, introducing competing high-affinity binding sites for DUX4 traps and prevents DUX4 from binding to its target genes. e, Although the exact mechanism of action of the p38 mitogen-activated protein kinase (MAPK) inhibitor losmapimod is unknown, this agent is proposed to reduce inflammation, possibly disrupting a feedforward loop that would otherwise increase DUX4 expression by increasing intracellular oxidative stress. SMCHD1, structural maintenance of chromosomes flexible hinge domain containing protein 1; UTR, untranslated region.
