Figure 6.
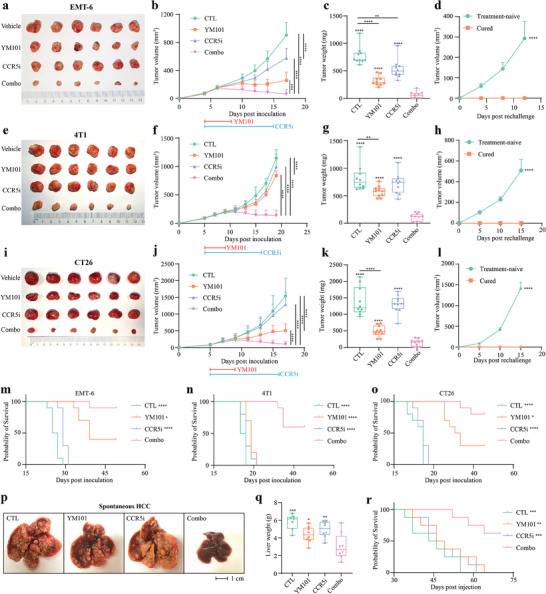
The synergistic antitumor effect between YM101 and CCR5 blockade. All mice were randomly assigned to one of four groups, namely the control, Maraviroc (CCR5i), YM101, and Maraviroc combined with YM101. Treatment was started when tumor volumes reached 50 mm3. Maraviroc (50 mg kg−1) was administered daily by intraperitoneal injection. For antibody treatments, tumor‐bearing mice received equimolar quantities of hIgG (6.6 mg kg−1) or YM101 (9 mg kg−1) every two days via intraperitoneal injection. The blue line indicates Maraviroc treatment, while the red line represents antibody treatment. Mice were euthanized when tumor volumes reached 2000 mm3 or at the end of experiments. a–c) Efficacy evaluation in the murine breast cancer EMT‐6 model (n = 10). d) Rechallenge assay in the EMT‐6 model (n = 4). e–g) Efficacy evaluation in the murine breast cancer 4T1 model (n = 10). h) Rechallenge assay in the 4T1 model (n = 2). i–k) Efficacy evaluation in the murine colon cancer CT26 model (n = 10). l) Rechallenge assay in the CT26 model (n = 2). m–o) The overall survival curves of EMT‐6, 4T1, and CT26 models (n = 10). p,q) Tumor burden of spontaneous hepatocellular carcinoma (HCC) model. Surviving tumor‐bearing mice were euthanized, and their livers were harvested 35 days after plasmid injection. The representative images of mouse livers were shown (n = 8; note: one mouse in the CTL group died before liver collection at the end of the experiment). r) The overall survival curves of spontaneous HCC model (n = 8). Statistical analyses were conducted using Student's t‐test (except for survival analysis) and Log‐rank test (for survival analysis). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 indicate significant differences compared to the Maraviroc combined with YM101 group.
