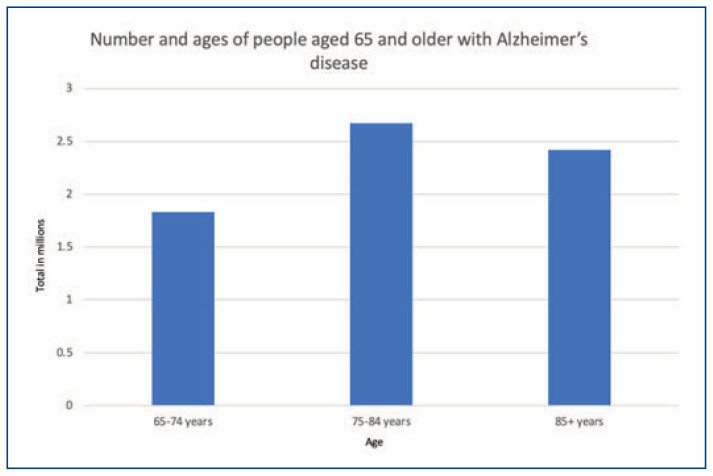
Alzheimer’s disease (AD), a neurodegenerative disease, afflicts approximately seven million people in the United States as of 2024 (Figure 1).1 This chronic, progressive disorder can only be definitively diagnosed on autopsy by directly observing characteristic atrophy in the cerebral cortex as well as amyloid-beta (Aβ) plaques and neurofibrillary tangles via microscopy. Without an autopsy, Alzheimer’s disease can be suspected based on clinical findings. Other conditions that can present similarly must be ruled out and features are identified via an accurate history, physical exam, bloodwork and neuroimaging in clinical settings, often primary care being the first point of contact. Additionally, cognitive tests and supportive imaging/lab testing require a clinical indication to perform them in the first place: oftentimes the emergence of symptoms or family history. Cognitive tests such as the Saint Louis University Mental Status (SLUMS) exam, the Mini Mental Status Exam (MMSE) and Montreal Cognitive Assessment (MoCA) are efficient and widely used tools to screen for cognitive impairment however are limited by language, education, and lower sensitivity for mild cognitive impairment (MCI).
Figure 1.
Prevalence of Alzheimer’s disease in 2024 by age.
Current Federally Approved Supportive Modalities for Alzheimer’s Disease Diagnosis
Imaging modalities such as magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography (PET) are frequently utilized and validated to aid a probable diagnosis. 18F fluoro-deoxy-glucose (FDG)-PET imaging, which measures cerebral glucose metabolism, has shown vbenefit in distinguishing mild cognitive impairment as it relates to AD from other neurodegenerative diseases and healthy controls. However, FDG-PET imaging has limitations in accuracy when it comes to older patients with late-onset AD. This led to the development of amyloid precursors for PET imaging as the more specific biomarkers for AD. Radiopharmaceuticals such as 11C-labeled Pittsburgh Compound-B (used in the first amyloid human amyloid PET study, but limited by a short half-life), [18F]Florbetapir and [18F]Florbetaben enable the quantification of cortical Aβ plaque burden and have a high specificity for the detection of AD pathophysiology.2 The newer radiotracers are taken up rapidly through the blood brain barrier and quickly washed out from areas that do not contain Aβ, allowing a clear contrast for cerebral amyloid burden. A limitation of the well-studied [18F]Florbetapir scans is that amyloid burden may be detected even in cognitively intact older individuals,3 especially those who have uncontrolled hypertension or who are carriers of the APOE4 gene (genetic variant known for risk of developing AD). Tau misfolding (a hallmark of Alzheimer’s pathology) can also be detected via PET imaging, for example via the United States Food and Drug Administration (FDA) approved radiotracer Flortaucipir.4 However, it is posited that this agent is a better diagnostic tool in people with more advanced stages of dementia rather than mild cognitive impairment. The utility of brain PET in general is limited by cost and availability to the patient.
The standard of care relative to the use of biomarkers to support a diagnosis of mild cognitive impairment due to AD includes amyloid PET or cerebrospinal fluid (CSF) examination. CSF biomarkers, specifically reduced amyloid-beta 42, elevated total tau and elevated phosphorylated tau, have been recognized since 2010s as important supporting evidence for early detection of AD. However, obtaining these samples still involves an invasive procedure (the lumbar puncture). This led to a growing need to discover blood/plasma-based biomarkers that may similarly aid diagnoses but are easier to obtain in primary care settings and can be used as preliminary screens to see if more expensive/invasive testing is indicated.
Developments in Blood-Based Biomarker Testing
In August 2019, a cohort study published in Neurology concluded the utility of plasma amyloid-beta 42/40 ratio in predicting current and future amyloidosis.5 The authors used an immunoprecipitation and liquid chromatography-mass spectrometry assay to measure this ratio in both plasma and CSF samples from 158 cognitively intact individuals. They compared the ratio to each person’s amyloid PET scan, which was the reference standard for current amyloidosis. They found high, statistically significant correspondence between plasma ratio and amyloid burden; in addition, they found that individuals with a positive amyloid-beta 42/40 ratio, but a negative amyloid PET scan, were at a 15-fold risk to convert to amyloid PET positive. Given this, the functional prospects of measuring a serum amyloid-beta 42/40 ratio to predict amyloid burden and in turn the development of AD were promising. The study also concluded that APOE4 status and age can improve the accuracy of utilizing this test. However, it is worth noting that an important limitation in the study was that the correspondence between the plasma amyloid-beta 42/40 ratio and cognitive impairment was not studied. In other words, the idea that this test will accurately predict a transition to cognitive impairment has not been studied.
Quest Diagnostics released the AD-Detect™ Test on July 31, 2023, a direct-to-consumer plasma-based biomarker test for AD that measures amyloid-beta 42 and amyloid-beta 40 in blood to provide an amyloid-beta 42/40 ratio.6 It was marketed for people aged 18 and older who may have mild cognitive impairment or a family history of AD. It could be purchased directly from the Quest website followed by scheduling an appointment at Quest labs. A telehealth physician would then review the purchase for medical necessity per the website, however it was not clear what the exact criteria to determine this necessity were. The results would then be made available online. Should the test be positive, a physician would contact the individual to talk about further steps. Broadly, Quest reported that the goal was to create an accessible, patient-initiated test that may be able to predict the risk for Alzheimer’s disease in patients with only mild symptoms. However, this test has not been approved by the FDA.
According to the Quest website, the AD-Detect™ Test was set to cost $399. It was not reimbursed by insurance. The results were to be provided in a numeric fashion along with distinct categories of low, medium, and high risk of amyloid presence in the brain. In addition, the website stated a report would be provided to patients of their current and past 5 amyloid-beta 42/40 ratios for patients to trend their risk. Per the Quest press release7 on July 31, 2023, a licensed physician would be reviewing website orders for the test to determine “appropriateness,” however the exact criteria for appropriateness and the specialty of the physician were not made clear. In addition, the press release also mentioned the option for individuals to discuss their results with a licensed physician, however the specifics of results interpretation and discussion were not made clear.
Due to the lack of long term, large scale clinical trials that can support the use of the AD-Detect™ test to conclusively guide assessment and treatment, it is not recommended for routine use in clinical settings. In addition, the Alzheimer’s Association did not endorse the use of the AD-Detect™ test by consumers given the lack of rigorous data and FDA approval.8 No peer-reviewed research validating the AD-Detect™ test had been published except an abstract of a poster presented at the Alzheimer’s Association International Conference; however, Quest then noted some of the data presented was incorrect and that the assay had a specificity of 71% for detecting PET-positivity.9 Experts then raised the concern that this number was not reassuring enough given the downstream impact of false positives attached to a test such as this.
The consequences of asymptomatic people accessing direct-to-consumer blood-based biomarker tests need to be considered carefully. Given this test is not currently considered evidence-based medicine, interpreting results can be confusing both for the patient as well as their physician. In addition, if an asymptomatic individual screened positive on the test, they may still not be eligible for current, FDA-approved AD treatments. Lifestyle modification may be the only advice that can be made to an asymptomatic individual who is deemed “intermediate” or “high” risk for amyloid burden, however it can be argued that these recommendations should be followed by all individuals anyway (better diets, exercise, controlling cardiovascular risk factors, keeping the mind active, etc). It is also worth noting that the presence of cerebral amyloid deposition does not always correlate with the development of cognitive impairment and may be related to normal aging and APOE genotype.10 Ethically, given the cost of this test and limited insurance coverage, the accessibility of this test may already be limited to individuals of certain financial means. The question also arises of how an individual who has been categorized as “intermediate” or “high” risk is supposed to use that information in their day-to-day functioning—should the individual be cognizant of their risk if they are working a high-stress, highly intellectualized job? Should they feel compelled to let family, friends, and employers know about their risk even if they have not manifested any symptoms? And what role should their clinicians, who did not directly recommend or administer the test, be playing for their patients? Dementia is still a highly stigmatized illness; the psychological ramifications of utilizing direct-to-consumer tests are not yet fully understood.
On July 19 2023, the University of Michigan published the results of a poll of people aged 65–80 and their thoughts relative to various tests to screen for cognitive impairment/disorders.11 Specifically for blood-based biomarkers, only 17% said they were familiar with such tests and 80% of older adults stated that they look to their health care providers for cognitive screening or blood-based biomarker tests if they feel they are appropriate. A majority of older individuals (74%) felt that a positive blood biomarker test result would make them believe they were likely to develop Alzheimer’s disease. Remarkably, the poll also found that 60% of older adults, women more than men, would experience significant distress in response to a positive result on either cognitive screening tests or blood tests. The responsibility of diagnosing a progressive illness such as AD should weigh heavily on clinicians determining the need to administer these tests; but for a direct-to-consumer test, these numbers highlight the potential for misunderstanding results if not guided carefully.
As of March 2024, AD-Detect™ is no longer being marketed on the Quest website for unclear reasons. It is also unclear if this withdrawal is temporary.
Another leading candidate for blood-based biomarker testing is phosphorylated tau 217 (p-tau217). A systematic review published in 2022 utilizing 676 publications showed that p-tau217 is a sensitive marker of manifestation and progression of AD and is specific to AD alone as it does not predict these changes in other neurodegenerative diseases.12 Previous studies have shown 100–400% increase in phosphorylated tau in patients with Alzheimer’s disease;13 this specific biomarker also correlates the most to an amyloid burden. Another study14 showed that plasma p-tau217 and phosphorylated tau 231 (p-tau231) had the strongest association with Aβ PET retention in early accumulating regions in preclinical Alzheimer’s disease. The PrecivityAD2, marketed by Washington University startup C2N Diagnostics, is a promising algorithm that combines plasma measures of percent p-tau217 with amyloid-beta 42/40 ratio. In a recent study15 involving 583 individuals with suspected AD, PrecivityAD2 yielded 88% agreement with amyloid PET and showed strong clinical validity across two independent cohorts. This test, like most other blood-based biomarker tests, is expensive and not reimbursed by insurance. At the time of this writing, there are no FDA-approved blood-based biomarkers.
Towards a New Definition of Alzheimer’s Disease?
The standard of care at this time for diagnosing AD is clinical; with the advent of blood-based biomarkers, there has been discussion about how this may change the very definition of AD diagnosis. A currently active National Institute on Aging and Alzheimer’s Association (NIA-AA) working group posits revising criteria for AD diagnosis from a clinical one to one strictly characterized by the objective presence of biomarkers.16 Per Dr. Clifford R. Jack Jr., the chair of this group, as quoted in a recent New York Times article, the definition of AD should be changed to mean “someone who has biomarker evidence of amyloid in the brain has the disease, whether they’re symptomatic or not”.17 This group does not recommend utilizing biomarkers for someone who does not have cognitive decline. However, this is likely to happen inadvertently and inevitably (especially given direct-to-consumer biomarker tests being marketed) and may lead to a diagnosis of AD without any associated clinical signs or symptoms. There are strong ethical concerns regarding asymptomatic individuals being diagnosed with AD and how they are expected to use this information— as mentioned earlier, should they be restricted from certain jobs, inform their friends and family, and suffer the significant distress of being diagnosed with AD when there is no absolute certainty that they will develop cognitive decline? Per the article, the American Geriatrics Society has already called the proposed idea “premature” and pointed out potential conflicts of interest due to several of the involved panel members having ties to pharmaceutical/biomarker companies.
Treatment Based on Biomarker Testing
Discussion regarding the drive to develop blood-based biomarker testing to detect cognitive impairment due to AD would be incomplete without mentioning the role of newer AD treatments. The argument has been made by proponents of using biomarker testing as the gold standard for AD diagnosis that clinicians can then start treatment with medications indicated for MCI due to AD and early stages of AD dementia. Specifically, aducanumab and lecanemab—these monoclonal antibodies administered via IV infusion target Aβ plaques. Aducanumab received accelerated FDA approval (not full FDA approval) but not without intense controversy. Lecanemab has received full FDA approval. Both drugs are indicated for mild cognitive impairment due to AD and mild AD. Biogen has since abandoned aducanumab.
In a phase III trial of lecanemab with 1,795 patients aged 50–90 years with MCI due to AD or early AD dementia AD, lecanemab slowed the rate of clinical decline by 27% after 18 months of treatment compared to placebo, leading to the authors concluding that “lecanemab reduced markers of amyloid in early Alzheimer’s disease and resulted in moderately less decline on measures of cognition and function than placebo at 18 months but was associated with adverse events.”18 One major potential side effect of these monoclonal antibodies is amyloid-related imaging abnormalities (ARIA), which most commonly presents as temporary swelling of the brain and may be accompanied by small areas of hemorrhage in or on the surface of the brain. It is often asymptomatic, but some people may have symptoms such as headache, confusion, and even seizures.19 These are not insignificant risks and need to be weighed versus potential clinical benefits.
Currently, a major four-year phase III clinical trial is underway called the AHEAD study which investigates whether administering lecanemab to amyloid-positive people who have no symptoms can prevent cognitive decline.20 Healthy adults aged 55–80 and without a prior diagnosis of AD are eligible for the study and individuals are divided to two separate sister trials depending on their baseline amyloid PET burden. Both trials utilize PET, plasma biomarker testing including PrecivityAD2, and CSF biomarker testing for outcome measures. Primary outcome measure for one of the two sister trials is cognitive testing and the other is amyloid PET.
Retinal Scans as Screening Tools
Emerging research is targeting other potential screening tools for Alzheimer’s disease including retinal scans. According to recent studies,21 optical coherence tomography angiography (OCTA) scans can be used to detect reduced capillary density and other retinal changes in APOE4 carriers. It was also discovered by the team that the capillary abnormalities were independent of amyloid pathology as suggested by prior PET scans. APOE4 variant has long been known to increase the risk of developing AD however new research just published22 has shown APOE4 homozygosity may be a true genetic cause of AD. The study, conducted in over 500 people with APOE4 homozygosity, showed by age 55, over 95% of them had AD related brain changes and by age 65, most of them had abnormal amyloid plaque accumulation. Detecting abnormalities that may be specific to APOE4 carriers in a non-invasive way may be very useful. However, it is important to note that the risk of ARIA with amyloid targeting drugs such as lecanemab is significantly elevated in individuals with two copies of APOE4, to the extent that some experts do not recommend this treatment for APOE4 homozygous individuals. The challenge remains—even if non-invasive tests can reasonably suggest the presence of APOE4, would the benefits of early treatment in those with mild cognitive impairment or no symptoms at all outweigh the risks?
Conclusion
Alzheimer’s disease screening and diagnosis remains an evolving field and is only set to become more complex. Robust evidence is still needed to justify routine use of emerging biomarkers, and in particular blood-based biomarkers, safely and effectively in the office setting. Thorough counseling, keeping in mind what is in the patient’s best interest, will be ever more important in selecting/utilizing biomarker testing for patients with cognitive impairment or at-risk for cognitive impairment due to conditions such as Alzheimer’s disease.
Footnotes
Vimita Patel, MD, (pictured), is a Resident in the Department of Psychiatry and Behavioral Neuroscience, and George Grossberg, MD, is the Henry and Amelia Nasrallah Professor, and Director, Division of Geriatric Psychiatry, Department of Psychiatry and Behavioral Neuroscience, Saint Louis University School of Medicine, St. Louis, Missouri.
Disclosure: No financial disclosures reported. Artificial intelligence was not used in the study, research, preparation, or writing of this manuscript.
References
- 1.Alzheimer’s Association. Alzheimer’s Disease Facts and Figures. Alzheimer’s Disease and Dementia. [Accessed May 10, 2024]. Available at: www.alz.org/alzheimers-dementia/facts-figures.
- 2.Suppiah S, Didier MA, Vinjamuri S. The who, when, why, and how of PET amyloid imaging in management of Alzheimer’s disease—Review of literature and interesting images. Diagnostics. 2019;9(2):65. doi: 10.3390/diagnostics9020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodrigue KM, Rieck JR, Kennedy KM, Devous MD, Diaz-Arrastia R, Park DC. Risk factors for β-amyloid deposition in healthy aging: vascular and genetic effects. JAMA Neurol. 2013;70(5):600–606. doi: 10.1001/jamaneurol.2013.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen GC, Roytman M, Chiang GC, Li Y, Gordon ML, Franceschi AM. Overview of tau PET molecular imaging. Curr Opin Neurol. 2022;35(2):230–239. doi: 10.1097/WCO.0000000000001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schindler SE, Bollinger JG, Ovod V, Mawuenyega KG, Li Y, Gordon BA, Bateman RJ. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019;93(17):e1647–e1659. doi: 10.1212/WNL.0000000000008081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quest Diagnostics. Quest Diagnostics: Test Directory. Quest Diagnostics; Oct, 2023. Quest AD-Detect, Beta-Amyloid 42/40 Ratio, Plasma. testdirectory.questdiagnostics.com/test/test-guides/TS_AD_Detect_BetaRatioPlasma/quest-ad-detect . [Google Scholar]
- 7.Quest Diagnostics. PR Newswire: Press Release Distribution, Targeting, Monitoring and Marketing. PR Newswire; Jul 31, 2023. Quest Introduces First-to-Market Consumer-Initiated Blood Test for Alzheimer’s Disease Risk Assessment on Questhealth.Com. Available at: www.prnewswire.com/news-releases/quest-introduces-first-to-marketconsumer-initiated-blood-test-for-alzheimers-disease-risk-assessment-onquesthealthcom301888.653.html. [Google Scholar]
- 8.George J. Quest’s Alzheimer’s Blood Test Has Experts Concerned. Medical News, MedpageToday. Aug 7, 2023. Available at: www.medpagetoday.com/neurology/alzheimersdisease/105784.
- 9.A new LC-MS/MS assay for the quantification of Aβ40 and A β ⊠ 42 in plasma: Validation and clinical performance. Alzheimers Dement. 2023;19:4771–4771. doi: 10.1002/alz.13443. [DOI] [Google Scholar]
- 10.Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR Amyloid Biomarker Study Group. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313(19):1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.University of Michigan School of Public Health. Early Signs of Alzheimer’s: Most Older Adults See the Value of Screening but Haven’t Been Tested: News. University of Michigan School of Public Health: Michigan Medicine: Dementia: Alzheimer’s: University of Michigan National Poll on Healthy Aging; Published July 19, 2023. Available at: sph.umich.edu/news/2023posts/early-signs-alzheimers-most-older-adults-see-value-screening-havent-been-tested.html. [Google Scholar]
- 12.Telser J, Risch L, Saely CH, Grossmann K, Werner P. P-tau217 in Alzheimer’s disease. Clin Chim Acta. 2022;531:100–111. doi: 10.1016/j.cca.2022.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Ashton NJ, Puig-Pijoan A, Milà-Alomà M, Fernández-Lebrero A, García-Escobar G, González-Ortiz F, Suárez-Calvet M. Plasma and CSF biomarkers in a memory clinic: head-to-head comparison of phosphorylated tau immunoassays. Alzheimers Dement. 2023;19(5):1913–1924. doi: 10.1002/alz.12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milà-Alomà M, Ashton NJ, Shekari M, Salvadó G, Ortiz-Romero P, Montoliu-Gaya L, Blennow K. Plasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer’s disease. Nat Med. 2022;28(9):1797–1801. doi: 10.1038/s41591-022-01925-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer MR, Kirmess KM, Eastwood S, et al. Clinical validation of the PrecivityAD2 blood test: A mass spectrometry-based test with algorithm combining %p-tau217 and Aβ42/40 ratio to identify presence of brain amyloid. Alzheimer’s Dement. 2024 doi: 10.1002/alz.13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jack CR, Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R Contributors. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Span P. Apparently healthy, but diagnosed with Alzheimer’s? The New York Times; Published March 4, 2024. Available at: www.nytimes.com/2024/03/04/health/alzheimers-amyloid-diagnosis.html. [Google Scholar]
- 18.Van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, Iwatsubo T. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9–21. doi: 10.1056/NEJMoa2212948. [DOI] [PubMed] [Google Scholar]
- 19.Office of the Commissioner. FDA converts novel Alzheimer’s disease treatment to traditional approval. U.S. Food and Drug Administration; Published July 6, 2023. Available at: www.fda.gov/news-events/press-announcements/fda-converts-novel-alzheimers-disease-treatment-traditional-approval. [Google Scholar]
- 20.Rafii MS, Sperling RA, Donohue MC, et al. The AHEAD 3-45 study: design of a prevention trial for Alzheimer’s disease. Alzheimer’s Dement. 2023;19(4):1227–1233. doi: 10.1002/alz.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin JY, Choi EY, Kim M, Lee HK, Byeon SH. Changes in retinal microvasculature and retinal layer thickness in association with apolipoprotein E genotype in Alzheimer’s disease. Sci Rep. 2021;1847;11(1) doi: 10.1038/s41598-020-80892-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fortea J, Pegueroles J, Alcolea D, Belbin O, Dols-Icardo O, Vaqué-Alcázar L, Montal V. APOE4 homozygosity represents a distinct genetic form of Alzheimer’s disease. Nat Med. 2024:1–8. doi: 10.1038/s41591-024-02931-w. [DOI] [PubMed] [Google Scholar]